Page 285 - Adsorbents fundamentals and applications
P. 285
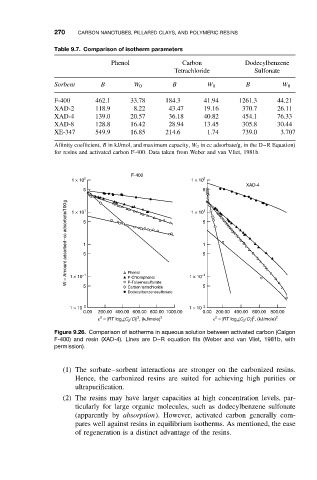
270 CARBON NANOTUBES, PILLARED CLAYS, AND POLYMERIC RESINS
Table 9.7. Comparison of isotherm parameters
Phenol Carbon Dodecylbenzene
Tetrachloride Sulfonate
Sorbent B W 0 B W 0 B W 0
F-400 462.1 33.78 184.3 41.94 1261.3 44.21
XAD-2 118.9 8.22 43.47 19.16 370.7 26.11
XAD-4 139.0 20.57 36.18 40.82 454.1 76.33
XAD-8 128.8 16.42 28.94 13.45 305.8 30.44
XE-347 549.9 16.85 214.6 1.74 739.0 3.707
Affinity coefficient, B in kJ/mol, and maximum capacity, W 0 in cc adsorbate/g, in the D–R Equation)
for resins and activated carbon F-400. Data taken from Weber and van Vliet, 1981b.
F-400
1 × 10 2 1 × 10 2
XAD-4
5 1 1 × 10 5 1
W = Amount adsorbed–cc adsorbate/100 g 5 1 5 5 1 5
1 × 10
−1
1 × 10
P-Chlorophenol
P-Toluenesulfonate
5 Phenol 1 × 10 −1 5
Carbon tetrachloride
Dodecylbenzenesulfonate
1 × 10 −2 1 × 10 −2
0.00 200.00 400.00 600.00 800.00 1000.00 0.00 200.00 400.00 600.00 800.00
∋ 2 = |RT log n (C 2 /C)| , (kJ/mole) 2 2 ∋ = |RT log n (C 2 /C)| , (kJ/mole) 2
2
2
Figure 9.26. Comparison of isotherms in aqueous solution between activated carbon (Calgon
F-400) and resin (XAD-4). Lines are D–R equation fits (Weber and van Vliet, 1981b, with
permission).
(1) The sorbate–sorbent interactions are stronger on the carbonized resins.
Hence, the carbonized resins are suited for achieving high purities or
ultrapurification.
(2) The resins may have larger capacities at high concentration levels, par-
ticularly for large organic molecules, such as dodecylbenzene sulfonate
(apparently by absorption). However, activated carbon generally com-
pares well against resins in equilibrium isotherms. As mentioned, the ease
of regeneration is a distinct advantage of the resins.