Page 280 - Adsorbents fundamentals and applications
P. 280
POLYMERIC RESINS 265
293 K Closed symbol : Ethylene
303 K
1.5 313 K Open symbol : Ethane
333 K
363 K
303 K Ethylene (unsupported clay)
303 K Ethane (unsupported clay)
1.0
q, m mol/g
0.5
0.0
0 200 400 600 800 1000 1200
P, mmHg
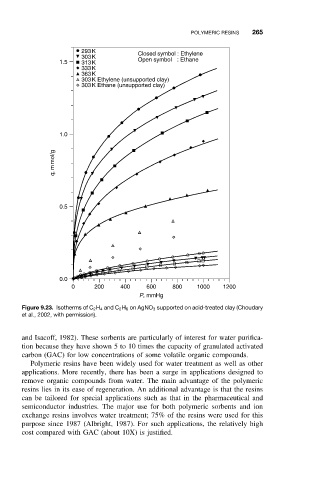
Figure 9.23. Isotherms of C 2 H 4 and C 2 H 6 on AgNO 3 supported on acid-treated clay (Choudary
et al., 2002, with permission).
and Isacoff, 1982). These sorbents are particularly of interest for water purifica-
tion because they have shown 5 to 10 times the capacity of granulated activated
carbon (GAC) for low concentrations of some volatile organic compounds.
Polymeric resins have been widely used for water treatment as well as other
applications. More recently, there has been a surge in applications designed to
remove organic compounds from water. The main advantage of the polymeric
resins lies in its ease of regeneration. An additional advantage is that the resins
can be tailored for special applications such as that in the pharmaceutical and
semiconductor industries. The major use for both polymeric sorbents and ion
exchange resins involves water treatment; 75% of the resins were used for this
purpose since 1987 (Albright, 1987). For such applications, the relatively high
cost compared with GAC (about 10X) is justified.