Page 278 - Adsorbents fundamentals and applications
P. 278
PILLARED CLAYS 263
1.0
A
0.8 B
Amount adsorbed (mmol/g) 0.6 a
b
0.4
0.2
0.0
0.0 0.2 0.4 0.6 0.8 1.0
P (atm)
◦
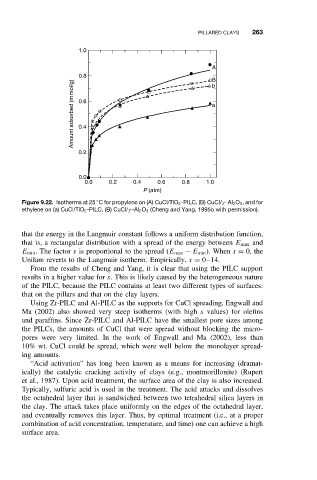
Figure 9.22. Isotherms at 25 C for propylene on (A) CuCl/TiO 2 -PILC, (B) CuCl/γ -Al 2 O 3 ,and for
ethylene on (a) CuCl/TiO 2 -PILC, (B) CuCl/γ -Al 2 O 3 (Cheng and Yang, 1995b with permission).
that the energy in the Langmuir constant follows a uniform distribution function,
that is, a rectangular distribution with a spread of the energy between E max and
E min . The factor s is proportional to the spread (E max − E min ). When s = 0, the
Unilam reverts to the Langmuir isotherm. Empirically, s = 0–14.
From the results of Cheng and Yang, it is clear that using the PILC support
results in a higher value for s. This is likely caused by the heterogeneous nature
of the PILC, because the PILC contains at least two different types of surfaces:
that on the pillars and that on the clay layers.
Using Zr-PILC and Al-PILC as the supports for CuCl spreading, Engwall and
Ma (2002) also showed very steep isotherms (with high s values) for olefins
and paraffins. Since Zr-PILC and Al-PILC have the smallest pore sizes among
the PILCs, the amounts of CuCl that were spread without blocking the micro-
pores were very limited. In the work of Engwall and Ma (2002), less than
10% wt. CuCl could be spread, which were well below the monolayer spread-
ing amounts.
“Acid activation” has long been known as a means for increasing (dramat-
ically) the catalytic cracking activity of clays (e.g., montmorillonite) (Rupert
et al., 1987). Upon acid treatment, the surface area of the clay is also increased.
Typically, sulfuric acid is used in the treatment. The acid attacks and dissolves
the octahedral layer that is sandwiched between two tetrahedral silica layers in
the clay. The attack takes place uniformly on the edges of the octahedral layer,
and eventually removes this layer. Thus, by optimal treatment (i.e., at a proper
combination of acid concentration, temperature, and time) one can achieve a high
surface area.