Page 274 - Adsorbents fundamentals and applications
P. 274
PILLARED CLAYS 259
1.0 1.0
Al 2 O _PILC Al O _PILC
3
3
2
0.9 Na_PILC 0.9 Ca_PILC
Differential pore volume (mL/g-nm) 0.7 Differential pore volume (mL/g-nm) 0.7
K_PILC
0.8
0.8
Cs_PILC
0.6
0.6
0.5
0.5
0.4
0.4
0.3
0.3
0.2
0.1 0.2
0.1
0.0 0.0
0.3 0.4 0.5 0.6 0.7 0.3 0.4 0.5 0.6 0.7
Pore width (nm) Pore width (nm)
(a) (b)
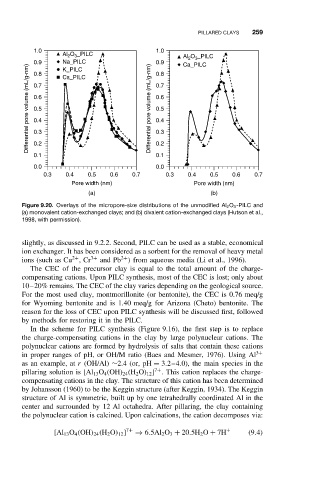
Figure 9.20. Overlays of the micropore-size distributions of the unmodified Al 2 O 3 -PILC and
(a) monovalent cation-exchanged clays; and (b) divalent cation-exchanged clays (Hutson et al.,
1998, with permission).
slightly, as discussed in 9.2.2. Second, PILC can be used as a stable, economical
ion exchanger. It has been considered as a sorbent for the removal of heavy metal
2+
2+
ions (such as Cu ,Cr 3+ and Pb ) from aqueous media (Li et al., 1996).
The CEC of the precursor clay is equal to the total amount of the charge-
compensating cations. Upon PILC synthesis, most of the CEC is lost; only about
10–20% remains. The CEC of the clay varies depending on the geological source.
For the most used clay, montmorillonite (or bentonite), the CEC is 0.76 meq/g
for Wyoming bentonite and is 1.40 meq/g for Arizona (Cheto) bentonite. The
reason for the loss of CEC upon PILC synthesis will be discussed first, followed
by methods for restoring it in the PILC.
In the scheme for PILC synthesis (Figure 9.16), the first step is to replace
the charge-compensating cations in the clay by large polynuclear cations. The
polynuclear cations are formed by hydrolysis of salts that contain these cations
in proper ranges of pH, or OH/M ratio (Baes and Mesmer, 1976). Using Al 3+
as an example, at r (OH/Al) ∼2.4 (or, pH = 3.2–4.0), the main species in the
pillaring solution is [Al 13 O 4 (OH) 24 (H 2 O) 12 ] . This cation replaces the charge-
7+
compensating cations in the clay. The structure of this cation has been determined
by Johansson (1960) to be the Keggin structure (after Keggin, 1934). The Keggin
structure of Al is symmetric, built up by one tetrahedrally coordinated Al in the
center and surrounded by 12 Al octahedra. After pillaring, the clay containing
the polynuclear cation is calcined. Upon calcinations, the cation decomposes via:
[Al 13 O 4 (OH) 24 (H 2 O) 12 ] 7+ ⇒ 6.5Al 2 O 3 + 20.5H 2 O + 7H + (9.4)