Page 269 - Adsorbents fundamentals and applications
P. 269
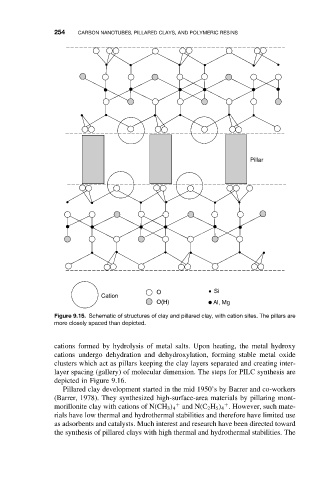
254 CARBON NANOTUBES, PILLARED CLAYS, AND POLYMERIC RESINS
Pillar
O Si
Cation
O(H) Al, Mg
Figure 9.15. Schematic of structures of clay and pillared clay, with cation sites. The pillars are
more closely spaced than depicted.
cations formed by hydrolysis of metal salts. Upon heating, the metal hydroxy
cations undergo dehydration and dehydroxylation, forming stable metal oxide
clusters which act as pillars keeping the clay layers separated and creating inter-
layer spacing (gallery) of molecular dimension. The steps for PILC synthesis are
depicted in Figure 9.16.
Pillared clay development started in the mid 1950’s by Barrer and co-workers
(Barrer, 1978). They synthesized high-surface-area materials by pillaring mont-
+
+
morillonite clay with cations of N(CH 3 ) 4 and N(C 2 H 5 ) 4 . However, such mate-
rials have low thermal and hydrothermal stabilities and therefore have limited use
as adsorbents and catalysts. Much interest and research have been directed toward
the synthesis of pillared clays with high thermal and hydrothermal stabilities. The