Page 272 - Adsorbents fundamentals and applications
P. 272
PILLARED CLAYS 257
(a)
(b)
(c)
(d)
(e)
4 5 6 7 8 9 10 11 12 13 14
2-Theta
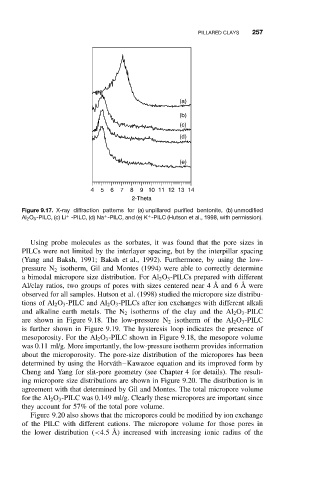
Figure 9.17. X-ray diffraction patterns for (a) unpillared purified bentonite, (b) unmodified
+
+
Al 2 O 3 -PILC, (c) Li -PILC, (d) Na -PILC, and (e) K -PILC (Hutson et al., 1998, with permission).
+
Using probe molecules as the sorbates, it was found that the pore sizes in
PILCs were not limited by the interlayer spacing, but by the interpillar spacing
(Yang and Baksh, 1991; Baksh et al., 1992). Furthermore, by using the low-
pressure N 2 isotherm, Gil and Montes (1994) were able to correctly determine
a bimodal micropore size distribution. For Al 2 O 3 -PILCs prepared with different
Al/clay ratios, two groups of pores with sizes centered near 4 ˚ Aand 6 ˚ Awere
observed for all samples. Hutson et al. (1998) studied the micropore size distribu-
tions of Al 2 O 3 -PILC and Al 2 O 3 -PILCs after ion exchanges with different alkali
and alkaline earth metals. The N 2 isotherms of the clay and the Al 2 O 3 -PILC
are shown in Figure 9.18. The low-pressure N 2 isotherm of the Al 2 O 3 -PILC
is further shown in Figure 9.19. The hysteresis loop indicates the presence of
mesoporosity. For the Al 2 O 3 -PILC shown in Figure 9.18, the mesopore volume
was 0.11 ml/g. More importantly, the low-pressure isotherm provides information
about the microporosity. The pore-size distribution of the micropores has been
determined by using the Horv´ ath–Kawazoe equation and its improved form by
Cheng and Yang for slit-pore geometry (see Chapter 4 for details). The result-
ing micropore size distributions are shown in Figure 9.20. The distribution is in
agreement with that determined by Gil and Montes. The total micropore volume
for the Al 2 O 3 -PILC was 0.149 ml/g. Clearly these micropores are important since
they account for 57% of the total pore volume.
Figure 9.20 also shows that the micropores could be modified by ion exchange
of the PILC with different cations. The micropore volume for those pores in
the lower distribution (<4.5 ˚ A) increased with increasing ionic radius of the