Page 271 - Adsorbents fundamentals and applications
P. 271
256 CARBON NANOTUBES, PILLARED CLAYS, AND POLYMERIC RESINS
hosts (Clearfield, 1988; Drezdon, 1988, Sprung et al., 1990; Burch, 1988; Van
Olphen, 1977; Fripiat, 1982,1988). In addition, several experimental parameters,
such as the concentration of the metal ion, the basicity or degree of hydrolysis
(given as r = OH/M), the temperature of preparation, the time and temperature
of aging, the type of counter-ion, and the method of preparation, can strongly
affect the degree of polymerization of the hydroxy-oligomeric cations in aqueous
solution (Burch, 1987), and consequently the physicochemical properties of the
pillared clays.
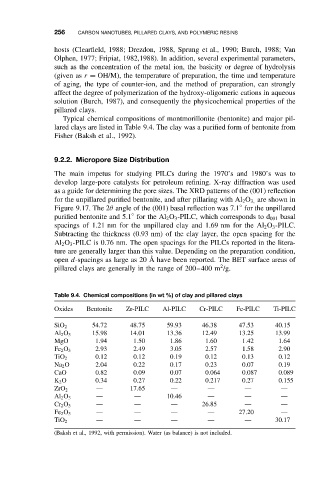
Typical chemical compositions of montmorillonite (bentonite) and major pil-
lared clays are listed in Table 9.4. The clay was a purified form of bentonite from
Fisher (Baksh et al., 1992).
9.2.2. Micropore Size Distribution
The main impetus for studying PILCs during the 1970’s and 1980’s was to
develop large-pore catalysts for petroleum refining. X-ray diffraction was used
as a guide for determining the pore sizes. The XRD patterns of the (001) reflection
for the unpillared purified bentonite, and after pillaring with Al 2 O 3, are shown in
◦
Figure 9.17. The 2θ angle of the (001) basal reflection was 7.1 for the unpillared
◦
purified bentonite and 5.1 for the Al 2 O 3 -PILC, which corresponds to d 001 basal
spacings of 1.21 nm for the unpillared clay and 1.69 nm for the Al 2 O 3 -PILC.
Subtracting the thickness (0.93 nm) of the clay layer, the open spacing for the
Al 2 O 3 -PILC is 0.76 nm. The open spacings for the PILCs reported in the litera-
ture are generally larger than this value. Depending on the preparation condition,
open d-spacings as large as 20 ˚ A have been reported. The BET surface areas of
2
pillared clays are generally in the range of 200–400 m /g.
Table 9.4. Chemical compositions (in wt %) of clay and pillared clays
Oxides Bentonite Zr-PILC Al-PILC Cr-PILC Fe-PILC Ti-PILC
SiO 2 54.72 48.75 59.93 46.38 47.53 40.15
Al 2 O 3 15.98 14.01 13.36 12.49 13.25 13.99
MgO 1.94 1.50 1.86 1.60 1.42 1.64
Fe 2 O 3 2.93 2.49 3.05 2.57 1.58 2.90
TiO 2 0.12 0.12 0.19 0.12 0.13 0.12
Na 2 O 2.04 0.22 0.17 0.23 0.07 0.19
CaO 0.82 0.09 0.07 0.064 0.087 0.089
K 2 O 0.34 0.27 0.22 0.217 0.27 0.155
ZrO 2 — 17.65 — — — —
Al 2 O 3 — — 10.46 — — —
Cr 2 O 3 — — — 26.85 — —
Fe 2 O 3 — — — — 27.20 —
TiO 2 — — — — — 30.17
(Baksh et al., 1992, with permission). Water (as balance) is not included.