Page 276 - Adsorbents fundamentals and applications
P. 276
PILLARED CLAYS 261
0.35 T=298K
BPL
0.30
Ti–PILC
Amount adsorbed, cm 3 STP/g 0.20 5A Fe–PILC
0.25
Cr–PILC
Al–PILC
Zr–PILC
0.15
0.10
0.05 FB
0.00
0.0 5.0 10.0 15.0 20.0 25.0
P, mmHg (torr)
◦
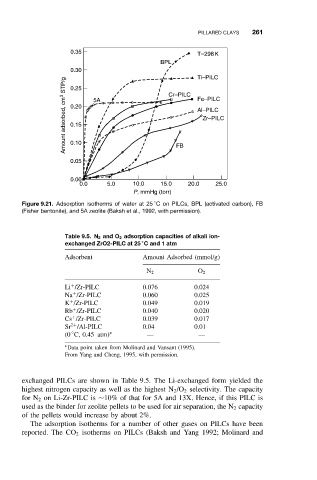
Figure 9.21. Adsorption isotherms of water at 25 C on PILCs, BPL (activated carbon), FB
(Fisher bentonite), and 5A zeolite (Baksh et al., 1992, with permission).
Table 9.5. N 2 and O 2 adsorption capacities of alkali ion-
exchanged ZrO2-PILC at 25 Cand1atm
◦
Adsorbent Amount Adsorbed (mmol/g)
N 2 O 2
+
Li /Zr-PILC 0.076 0.024
+
Na /Zr-PILC 0.060 0.025
+
K /Zr-PILC 0.049 0.019
+
Rb /Zr-PILC 0.040 0.020
+
Cs /Zr-PILC 0.039 0.017
Sr /Al-PILC 0.04 0.01
2+
◦
(0 C, 0.45 atm) ∗ — —
∗ Data point taken from Molinard and Vansant (1995).
From Yang and Cheng, 1995, with permission.
exchanged PILCs are shown in Table 9.5. The Li-exchanged form yielded the
highest nitrogen capacity as well as the highest N 2 /O 2 selectivity. The capacity
for N 2 on Li-Zr-PILC is ∼10% of that for 5A and 13X. Hence, if this PILC is
used as the binder for zeolite pellets to be used for air separation, the N 2 capacity
of the pellets would increase by about 2%.
The adsorption isotherms for a number of other gases on PILCs have been
reported. The CO 2 isotherms on PILCs (Baksh and Yang 1992; Molinard and