Page 282 - Adsorbents fundamentals and applications
P. 282
POLYMERIC RESINS 267
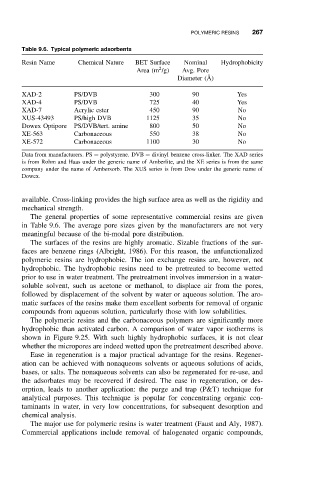
Table 9.6. Typical polymeric adsorbents
Resin Name Chemical Nature BET Surface Nominal Hydrophobicity
2
Area (m /g) Avg. Pore
Diameter ( ˚ A)
XAD-2 PS/DVB 300 90 Yes
XAD-4 PS/DVB 725 40 Yes
XAD-7 Acrylic ester 450 90 No
XUS-43493 PS/high DVB 1125 35 No
Dowex Optipore PS/DVB/tert. amine 800 50 No
XE-563 Carbonaceous 550 38 No
XE-572 Carbonaceous 1100 30 No
Data from manufacturers. PS = polystyrene. DVB = divinyl benzene cross-linker. The XAD series
is from Rohm and Haas under the generic name of Amberlite, and the XE series is from the same
company under the name of Ambersorb. The XUS series is from Dow under the generic name of
Dowex.
available. Cross-linking provides the high surface area as well as the rigidity and
mechanical strength.
The general properties of some representative commercial resins are given
in Table 9.6. The average pore sizes given by the manufacturers are not very
meaningful because of the bi-modal pore distribution.
The surfaces of the resins are highly aromatic. Sizable fractions of the sur-
faces are benzene rings (Albright, 1986). For this reason, the unfunctionalized
polymeric resins are hydrophobic. The ion exchange resins are, however, not
hydrophobic. The hydrophobic resins need to be pretreated to become wetted
prior to use in water treatment. The pretreatment involves immersion in a water-
soluble solvent, such as acetone or methanol, to displace air from the pores,
followed by displacement of the solvent by water or aqueous solution. The aro-
matic surfaces of the resins make them excellent sorbents for removal of organic
compounds from aqueous solution, particularly those with low solubilities.
The polymeric resins and the carbonaceous polymers are significantly more
hydrophobic than activated carbon. A comparison of water vapor isotherms is
shown in Figure 9.25. With such highly hydrophobic surfaces, it is not clear
whether the micropores are indeed wetted upon the pretreatment described above.
Ease in regeneration is a major practical advantage for the resins. Regener-
ation can be achieved with nonaqueous solvents or aqueous solutions of acids,
bases, or salts. The nonaqueous solvents can also be regenerated for re-use, and
the adsorbates may be recovered if desired. The ease in regeneration, or des-
orption, leads to another application: the purge and trap (P&T) technique for
analytical purposes. This technique is popular for concentrating organic con-
taminants in water, in very low concentrations, for subsequent desorption and
chemical analysis.
The major use for polymeric resins is water treatment (Faust and Aly, 1987).
Commercial applications include removal of halogenated organic compounds,