Page 297 - Adsorbents fundamentals and applications
P. 297
282 SORBENTS FOR APPLICATIONS
in some detail in this chapter. With the advances made in both sorbents and
PSA/VSA cycle technology, PSA/VSA accounts for ∼20% of the oxygen and
nitrogen, and the size of a single PSA/VSA unit is approaching 250 tons/day
oxygen, while these numbers continue to grow steadily. The sorbent productivity
(or bed inventory), often expressed as “bed-size factor” (Leavitt, 1992), is well
below 1000 lb of zeolite for per ton/day O 2 product (Notaro et al., 1999). The
total power consumption is below 250 kWh per ton O 2 .
10.1.1. 5A and 13X Zeolites
5A (CaA) and 13X (NaX) zeolites have been the most commonly used sorbents
for air separation. The N 2 /O 2 isotherms on 5A that are reported in the literature
vary widely, depending on the cation composition in the zeolite. The typical
commercial 5A used for air separation is made by exchanging ∼70% of the Na +
2+
in NaA by Ca .
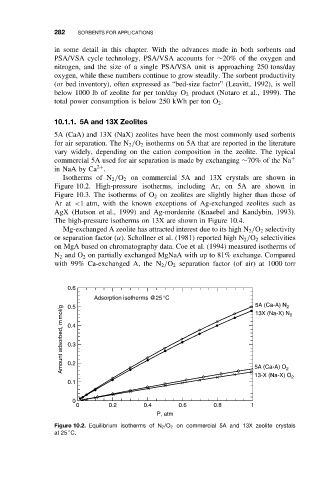
Isotherms of N 2 /O 2 on commercial 5A and 13X crystals are shown in
Figure 10.2. High-pressure isotherms, including Ar, on 5A are shown in
Figure 10.3. The isotherms of O 2 on zeolites are slightly higher than those of
Ar at <1 atm, with the known exceptions of Ag-exchanged zeolites such as
AgX (Hutson et al., 1999) and Ag-mordenite (Knaebel and Kandybin, 1993).
The high-pressure isotherms on 13X are shown in Figure 10.4.
Mg-exchanged A zeolite has attracted interest due to its high N 2 /O 2 selectivity
or separation factor (α). Schollner et al. (1981) reported high N 2 /O 2 selectivities
on MgA based on chromatography data. Coe et al. (1994) measured isotherms of
N 2 and O 2 on partially exchanged MgNaA with up to 81% exchange. Compared
with 99% Ca-exchanged A, the N 2 /O 2 separation factor (of air) at 1000 torr
0.6
Adsorption isotherms @25°C 5A (Ca-A) N 2 2
0.5
Amount adsorbed, m mol/g 0.4
13X (Na-X) N
0.3
0.2
5A (Ca-A) O
2
13-X (Na-X) O 2
0.1
0
0 0.2 0.4 0.6 0.8 1
P, atm
Figure 10.2. Equilibrium isotherms of N 2 /O 2 on commercial 5A and 13X zeolite crystals
◦
at 25 C.