Page 298 - Adsorbents fundamentals and applications
P. 298
AIR SEPARATION 283
10
9
8
Qty ads. (molecules/cavity) 6
7
5
4
3
2
1
0
0 1 2 3 4 5
Pressure (bar)
297.15K 233.15 K 203.15K
Nitrogen
Oxygen
Argon
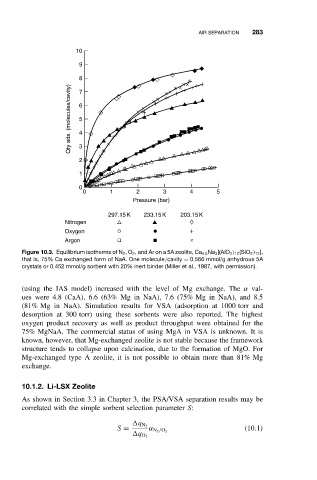
Figure 10.3. Equilibrium isotherms of N 2 ,O 2 , and Ar on a 5A zeolite, Ca 4.5 Na 3 [(AlO 2 ) 12 (SiO 2 ) 12 ],
that is, 75% Ca exchanged form of NaA. One molecule/cavity = 0.566 mmol/g anhydrous 5A
crystals or 0.452 mmol/g sorbent with 20% inert binder (Miller et al., 1987, with permission).
(using the IAS model) increased with the level of Mg exchange. The α val-
ues were 4.8 (CaA), 6.6 (63% Mg in NaA), 7.6 (75% Mg in NaA), and 8.5
(81% Mg in NaA). Simulation results for VSA (adsorption at 1000 torr and
desorption at 300 torr) using these sorbents were also reported. The highest
oxygen product recovery as well as product throughput were obtained for the
75% MgNaA. The commercial status of using MgA in VSA is unknown. It is
known, however, that Mg-exchanged zeolite is not stable because the framework
structure tends to collapse upon calcination, due to the formation of MgO. For
Mg-exchanged type A zeolite, it is not possible to obtain more than 81% Mg
exchange.
10.1.2. Li-LSX Zeolite
As shown in Section 3.3 in Chapter 3, the PSA/VSA separation results may be
correlated with the simple sorbent selection parameter S:
q N 2
S = α N 2 /O 2 (10.1)
q O 2