Page 299 - Adsorbents fundamentals and applications
P. 299
284 SORBENTS FOR APPLICATIONS
10
9
8
Qty ads. (molecules/cavity) 6
7
5
4
3
2
1
0
0 1 2 3 4 5
Pressure (bar)
323.15K 297.15K 233.15K 203.15K
Nitrogen
Oxygen
Argon
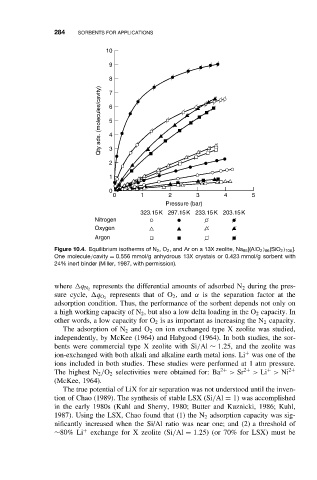
Figure 10.4. Equilibrium isotherms of N 2 ,O 2 , and Ar on a 13X zeolite, Na 86 [(AlO 2 ) 86 (SiO 2 ) 106 ].
One molecule/cavity = 0.556 mmol/g anhydrous 13X crystals or 0.423 mmol/g sorbent with
24% inert binder (Miller, 1987, with permission).
represents the differential amounts of adsorbed N 2 during the pres-
where q N 2
represents that of O 2 ,and α is the separation factor at the
sure cycle, q O 2
adsorption condition. Thus, the performance of the sorbent depends not only on
a high working capacity of N 2 , but also a low delta loading in the O 2 capacity. In
other words, a low capacity for O 2 is as important as increasing the N 2 capacity.
The adsorption of N 2 and O 2 on ion exchanged type X zeolite was studied,
independently, by McKee (1964) and Habgood (1964). In both studies, the sor-
bents were commercial type X zeolite with Si/Al ∼ 1.25, and the zeolite was
+
ion-exchanged with both alkali and alkaline earth metal ions. Li was one of the
ions included in both studies. These studies were performed at 1 atm pressure.
+
The highest N 2 /O 2 selectivities were obtained for: Ba 2+ > Sr 2+ > Li > Ni 2+
(McKee, 1964).
The true potential of LiX for air separation was not understood until the inven-
tion of Chao (1989). The synthesis of stable LSX (Si/Al = 1) was accomplished
in the early 1980s (Kuhl and Sherry, 1980; Butter and Kuznicki, 1986; Kuhl,
1987). Using the LSX, Chao found that (1) the N 2 adsorption capacity was sig-
nificantly increased when the Si/Al ratio was near one; and (2) a threshold of
∼80% Li + exchange for X zeolite (Si/Al = 1.25) (or 70% for LSX) must be