Page 303 - Adsorbents fundamentals and applications
P. 303
288 SORBENTS FOR APPLICATIONS
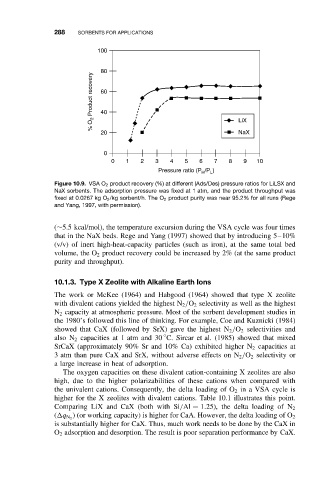
100
80
% O 2 Product recovery 60
40
20 LiX
NaX
0
0 1 2 3 4 5 6 7 8 9 10
Pressure ratio (P /P )
H
L
Figure 10.9. VSA O 2 product recovery (%) at different (Ads/Des) pressure ratios for LiLSX and
NaX sorbents. The adsorption pressure was fixed at 1 atm, and the product throughput was
fixed at 0.0267 kg O 2 /kg sorbent/h. The O 2 product purity was near 95.2% for all runs (Rege
and Yang, 1997, with permission).
(∼5.5 kcal/mol), the temperature excursion during the VSA cycle was four times
that in the NaX beds. Rege and Yang (1997) showed that by introducing 5–10%
(v/v) of inert high-heat-capacity particles (such as iron), at the same total bed
volume, the O 2 product recovery could be increased by 2% (at the same product
purity and throughput).
10.1.3. Type X Zeolite with Alkaline Earth Ions
The work or McKee (1964) and Habgood (1964) showed that type X zeolite
with divalent cations yielded the highest N 2 /O 2 selectivity as well as the highest
N 2 capacity at atmospheric pressure. Most of the sorbent development studies in
the 1980’s followed this line of thinking. For example, Coe and Kuznicki (1984)
showed that CaX (followed by SrX) gave the highest N 2 /O 2 selectivities and
◦
also N 2 capacities at 1 atm and 30 C. Sircar et al. (1985) showed that mixed
SrCaX (approximately 90% Sr and 10% Ca) exhibited higher N 2 capacities at
3 atm than pure CaX and SrX, without adverse effects on N 2 /O 2 selectivity or
a large increase in heat of adsorption.
The oxygen capacities on these divalent cation-containing X zeolites are also
high, due to the higher polarizabilities of these cations when compared with
the univalent cations. Consequently, the delta loading of O 2 in a VSA cycle is
higher for the X zeolites with divalent cations. Table 10.1 illustrates this point.
Comparing LiX and CaX (both with Si/Al = 1.25), the delta loading of N 2
) (or working capacity) is higher for CaA. However, the delta loading of O 2
( q N 2
is substantially higher for CaX. Thus, much work needs to be done by the CaX in
O 2 adsorption and desorption. The result is poor separation performance by CaX.