Page 306 - Adsorbents fundamentals and applications
P. 306
AIR SEPARATION 291
1985). It was found that, for dehydrated and fully Ag -exchanged faujasite-type
+
zeolites, the silver molecules were distributed among the six-ring sites (SI, SI ,
and SII for faujasites) and, for samples with high Al content, in the SIII loca-
tions. Gellens et al. (1981b) and Baker et al. (1985) showed the simultaneous
0
+
+
occupancy of sites SI and SI by linear (Ag − Ag − Ag ) clusters. Further
information (prior to 1994) can be found in a comprehensive review of silver
clusters and chemistry in zeolites by Sun and Seff (1994).
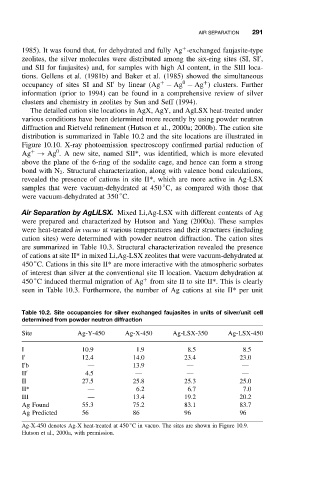
The detailed cation site locations in AgX, AgY, and AgLSX heat-treated under
various conditions have been determined more recently by using powder neutron
diffraction and Rietveld refinement (Hutson et al., 2000a; 2000b). The cation site
distribution is summarized in Table 10.2 and the site locations are illustrated in
Figure 10.10. X-ray photoemission spectroscopy confirmed partial reduction of
0
+
Ag → Ag . A new site, named SII*, was identified, which is more elevated
above the plane of the 6-ring of the sodalite cage, and hence can form a strong
bond with N 2 . Structural characterization, along with valence bond calculations,
revealed the presence of cations in site II*, which are more active in Ag-LSX
◦
samples that were vacuum-dehydrated at 450 C, as compared with those that
◦
were vacuum-dehydrated at 350 C.
Air Separation by AgLiLSX. Mixed Li,Ag-LSX with different contents of Ag
were prepared and characterized by Hutson and Yang (2000a). These samples
were heat-treated in vacuo at various temperatures and their structures (including
cation sites) were determined with powder neutron diffraction. The cation sites
are summarized in Table 10.3. Structural characterization revealed the presence
of cations at site II* in mixed Li,Ag-LSX zeolites that were vacuum-dehydrated at
◦
450 C. Cations in this site II* are more interactive with the atmospheric sorbates
of interest than silver at the conventional site II location. Vacuum dehydration at
◦
+
450 C induced thermal migration of Ag from site II to site II*. This is clearly
seen in Table 10.3. Furthermore, the number of Ag cations at site II* per unit
Table 10.2. Site occupancies for silver exchanged faujasites in units of silver/unit cell
determined from powder neutron diffraction
Site Ag-Y-450 Ag-X-450 Ag-LSX-350 Ag-LSX-450
I 10.9 1.9 8.5 8.5
I 12.4 14.0 23.4 23.0
I b — 13.9 — —
II 4.5 — — —
II 27.5 25.8 25.3 25.0
II* — 6.2 6.7 7.0
III — 13.4 19.2 20.2
Ag Found 55.3 75.2 83.1 83.7
Ag Predicted 56 86 96 96
◦
Ag-X-450 denotes Ag-X heat-treated at 450 C in vacuo. The sites are shown in Figure 10.9.
Hutson et al., 2000a, with permission.