Page 309 - Adsorbents fundamentals and applications
P. 309
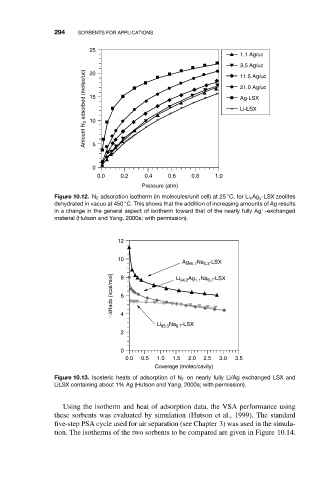
294 SORBENTS FOR APPLICATIONS
25
1.1 Ag/uc
3.5 Ag/uc
Amount N 2 adsorbed (molec/uc) 15 21.0 Ag/uc
20
11.5 Ag/uc
Ag-LSX
Li-LSX
10
0 5
0.0 0.2 0.4 0.6 0.8 1.0
Pressure (atm)
◦
Figure 10.12. N 2 adsorption isotherm (in molecules/unit cell) at 25 C, for Li x Ag y -LSX zeolites
◦
dehydrated in vacuo at 450 C. This shows that the addition of increasing amounts of Ag results
+
in a change in the general aspect of isotherm toward that of the nearly fully Ag -exchanged
material (Hutson and Yang, 2000a; with permission).
12
10
Ag 95.7 Na 0.3 -LSX
8
−ÐHads (kcal/mol) 6
Li 94.2 Ag 1.1 Na 0.7 -LSX
4
Li 95.3 Na 0.7 -LSX
2
0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5
Coverage (molec/cavity)
Figure 10.13. Isosteric heats of adsorption of N 2 on nearly fully Li/Ag exchanged LSX and
LiLSX containing about 1% Ag (Hutson and Yang, 2000a; with permission).
Using the isotherm and heat of adsorption data, the VSA performance using
these sorbents was evaluated by simulation (Hutson et al., 1999). The standard
five-step PSA cycle used for air separation (see Chapter 3) was used in the simula-
tion. The isotherms of the two sorbents to be compared are given in Figure 10.14.