Page 312 - Adsorbents fundamentals and applications
P. 312
AIR SEPARATION 297
the oxygen-carrying complexes of cobalt that have proven the most promising
as potential oxygen sorbents for air separation. Several thorough reviews are
available on this subject (Jones et al., 1979; Niederhoffer et al., 1994; Li and
Govind, 1994).
Pfeiffer described a compound of composition cobaltous bis-salicylaldehyde
ethylenediamine that turned from a reddish color to black when exposed to air
(Li and Govind, 1994). Tsumakei then showed that the blackening was due to
adsorption of oxygen from the air (Li and Govind, 1994). He also showed that
the sorption was reversible and that the oxygen could be driven off by heat-
ing in carbon dioxide. These results then stimulated a tremendous amount of
work by Calvin and co-workers who synthesized and characterized a large num-
ber of cobalt chelates capable of binding oxygen (Calvin et al., 1946; Bailes
et al., 1947; Calvin and Martell, 1952). Among these compounds were cobal-
tous bis-salicylaldehyde ethylenediamine (Co(salen) or salcomine) and cobaltous
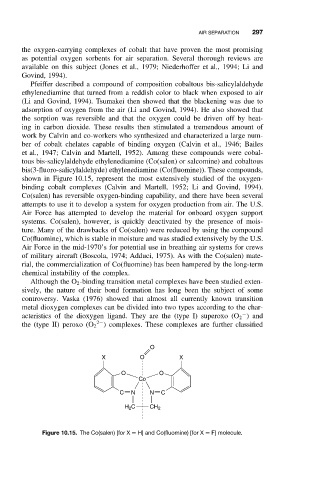
bis(3-fluoro-salicylaldehyde) ethylenediamine (Co(fluomine)). These compounds,
shown in Figure 10.15, represent the most extensively studied of the oxygen-
binding cobalt complexes (Calvin and Martell, 1952; Li and Govind, 1994).
Co(salen) has reversible oxygen-binding capability, and there have been several
attempts to use it to develop a system for oxygen production from air. The U.S.
Air Force has attempted to develop the material for onboard oxygen support
systems. Co(salen), however, is quickly deactivated by the presence of mois-
ture. Many of the drawbacks of Co(salen) were reduced by using the compound
Co(fluomine), which is stable in moisture and was studied extensively by the U.S.
Air Force in the mid-1970’s for potential use in breathing air systems for crews
of military aircraft (Boscola, 1974; Adduci, 1975). As with the Co(salen) mate-
rial, the commercialization of Co(fluomine) has been hampered by the long-term
chemical instability of the complex.
Although the O 2 -binding transition metal complexes have been studied exten-
sively, the nature of their bond formation has long been the subject of some
controversy. Vaska (1976) showed that almost all currently known transition
metal dioxygen complexes can be divided into two types according to the char-
acteristics of the dioxygen ligand. They are the (type I) superoxo (O 2 )and
−
2−
the (type II) peroxo (O 2 ) complexes. These complexes are further classified
O
X O X
O O
Co
Co
C N N C
H 2 C CH 2
Figure 10.15. The Co(salen) [for X = H] and Co(fluomine) [for X = F] molecule.