Page 315 - Adsorbents fundamentals and applications
P. 315
300 SORBENTS FOR APPLICATIONS
O 2 -binding capacity for the Co(salen) material was 1.06 mmol/g, approximately
2+
70% of the theoretical value of 1.55 mmol/g based on a 1 : 2 (O 2 :Co ) adduct.
◦
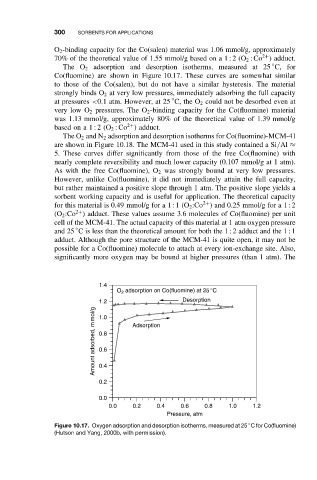
The O 2 adsorption and desorption isotherms, measured at 25 C, for
Co(fluomine) are shown in Figure 10.17. These curves are somewhat similar
to those of the Co(salen), but do not have a similar hysteresis. The material
strongly binds O 2 at very low pressures, immediately adsorbing the full capacity
◦
at pressures <0.1 atm. However, at 25 C, the O 2 could not be desorbed even at
very low O 2 pressures. The O 2 -binding capacity for the Co(fluomine) material
was 1.13 mmol/g, approximately 80% of the theoretical value of 1.39 mmol/g
basedon a 1 : 2(O 2 :Co ) adduct.
2+
The O 2 and N 2 adsorption and desorption isotherms for Co(fluomine)-MCM-41
are shown in Figure 10.18. The MCM-41 used in this study contained a Si/Al ≈
5. These curves differ significantly from those of the free Co(fluomine) with
nearly complete reversibility and much lower capacity (0.107 mmol/g at 1 atm).
As with the free Co(fluomine), O 2 was strongly bound at very low pressures.
However, unlike Co(fluomine), it did not immediately attain the full capacity,
but rather maintained a positive slope through 1 atm. The positive slope yields a
sorbent working capacity and is useful for application. The theoretical capacity
for this material is 0.49 mmol/g for a 1 : 1 (O 2 :Co ) and 0.25 mmol/g for a 1 : 2
2+
2+
(O 2 :Co ) adduct. These values assume 3.6 molecules of Co(fluomine) per unit
cell of the MCM-41. The actual capacity of this material at 1 atm oxygen pressure
◦
and 25 C is less than the theoretical amount for both the 1 : 2 adduct and the 1 : 1
adduct. Although the pore structure of the MCM-41 is quite open, it may not be
possible for a Co(fluomine) molecule to attach at every ion-exchange site. Also,
significantly more oxygen may be bound at higher pressures (than 1 atm). The
1.4
O adsorption on Co(fluomine) at 25°C
2
1.2 Desorption
Amount adsorbed, m mol/g 0.8 Adsorption
1.0
0.6
0.4
0.2
0.0
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Pressure, atm
◦
Figure 10.17. Oxygen adsorption and desorption isotherms, measured at 25 C for Co(fluomine)
(Hutson and Yang, 2000b, with permission).