Page 320 - Adsorbents fundamentals and applications
P. 320
HYDROGEN STORAGE 305
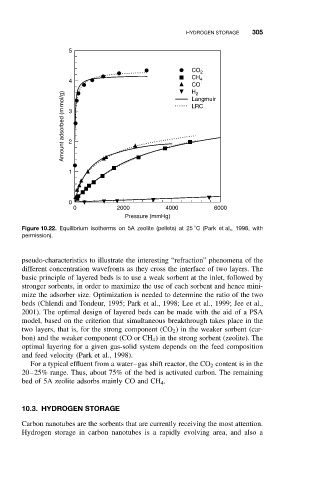
5
CO 2
CH 4
4
CO
H
Amount adsorbed (m mol/g) 3 2 LRC
2
Langmuir
1
0
0 2000 4000 6000
Pressure (mmHg)
◦
Figure 10.22. Equilibrium isotherms on 5A zeolite (pellets) at 25 C (Park et al., 1998, with
permission).
pseudo-characteristics to illustrate the interesting “refraction” phenomena of the
different concentration wavefronts as they cross the interface of two layers. The
basic principle of layered beds is to use a weak sorbent at the inlet, followed by
stronger sorbents, in order to maximize the use of each sorbent and hence mini-
mize the adsorber size. Optimization is needed to determine the ratio of the two
beds (Chlendi and Tondeur, 1995; Park et al., 1998; Lee et al., 1999; Jee et al.,
2001). The optimal design of layered beds can be made with the aid of a PSA
model, based on the criterion that simultaneous breakthrough takes place in the
two layers, that is, for the strong component (CO 2 ) in the weaker sorbent (car-
bon) and the weaker component (CO or CH 4 ) in the strong sorbent (zeolite). The
optimal layering for a given gas-solid system depends on the feed composition
and feed velocity (Park et al., 1998).
For a typical effluent from a water–gas shift reactor, the CO 2 content is in the
20–25% range. Thus, about 75% of the bed is activated carbon. The remaining
bed of 5A zeolite adsorbs mainly CO and CH 4 .
10.3. HYDROGEN STORAGE
Carbon nanotubes are the sorbents that are currently receiving the most attention.
Hydrogen storage in carbon nanotubes is a rapidly evolving area, and also a