Page 318 - Adsorbents fundamentals and applications
P. 318
HYDROGEN PURIFICATION 303
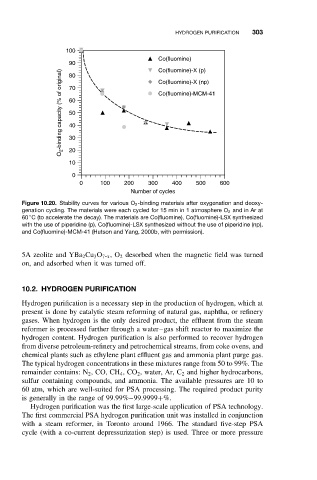
100
Co(fluomine)
90 Co(fluomine)-X (p)
O 2 -binding capacity (% of original) 60 Co(fluomine)-MCM-41
80
Co(fluomine)-X (np)
70
50
40
30
20
10
0
0 100 200 300 400 500 600
Number of cycles
Figure 10.20. Stability curves for various O 2 -binding materials after oxygenation and deoxy-
genation cycling. The materials were each cycled for 15 min in 1 atmosphere O 2 and in Ar at
◦
60 C (to accelerate the decay). The materials are Co(fluomine), Co(fluomine)-LSX synthesized
with the use of piperidine (p), Co(fluomine)-LSX synthesized without the use of piperidine (np),
and Co(fluomine)-MCM-41 (Hutson and Yang, 2000b, with permission).
5A zeolite and YBa 2 Cu 3 O 7-x ,O 2 desorbed when the magnetic field was turned
on, and adsorbed when it was turned off.
10.2. HYDROGEN PURIFICATION
Hydrogen purification is a necessary step in the production of hydrogen, which at
present is done by catalytic steam reforming of natural gas, naphtha, or refinery
gases. When hydrogen is the only desired product, the effluent from the steam
reformer is processed further through a water–gas shift reactor to maximize the
hydrogen content. Hydrogen purification is also performed to recover hydrogen
from diverse petroleum-refinery and petrochemical streams, from coke ovens, and
chemical plants such as ethylene plant effluent gas and ammonia plant purge gas.
The typical hydrogen concentrations in these mixtures range from 50 to 99%. The
remainder contains: N 2 ,CO, CH 4 ,CO 2 , water, Ar, C 2 and higher hydrocarbons,
sulfur containing compounds, and ammonia. The available pressures are 10 to
60 atm, which are well-suited for PSA processing. The required product purity
is generally in the range of 99.99%–99.9999+%.
Hydrogen purification was the first large-scale application of PSA technology.
The first commercial PSA hydrogen purification unit was installed in conjunction
with a steam reformer, in Toronto around 1966. The standard five-step PSA
cycle (with a co-current depressurization step) is used. Three or more pressure