Page 319 - Adsorbents fundamentals and applications
P. 319
304 SORBENTS FOR APPLICATIONS
equalization steps are employed to maximize the product recovery. A review of
the technology development prior to 1986 was given in Yang (1987).
Because of the wide range of adsorptive properties of the gas molecules in the
feed, it was recognized from the early development that more than one sorbent
was needed for the separation. Hence, layered beds were used from the beginning.
Typically, the first layer (at the feed end) is activated carbon, which is followed
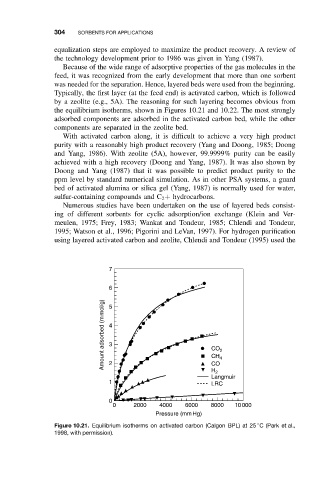
by a zeolite (e.g., 5A). The reasoning for such layering becomes obvious from
the equilibrium isotherms, shown in Figures 10.21 and 10.22. The most strongly
adsorbed components are adsorbed in the activated carbon bed, while the other
components are separated in the zeolite bed.
With activated carbon along, it is difficult to achieve a very high product
purity with a reasonably high product recovery (Yang and Doong, 1985; Doong
and Yang, 1986). With zeolite (5A), however, 99.9999% purity can be easily
achieved with a high recovery (Doong and Yang, 1987). It was also shown by
Doong and Yang (1987) that it was possible to predict product purity to the
ppm level by standard numerical simulation. As in other PSA systems, a guard
bed of activated alumina or silica gel (Yang, 1987) is normally used for water,
sulfur-containing compounds and C 2 + hydrocarbons.
Numerous studies have been undertaken on the use of layered beds consist-
ing of different sorbents for cyclic adsorption/ion exchange (Klein and Ver-
meulen, 1975; Frey, 1983; Wankat and Tondeur, 1985; Chlendi and Tondeur,
1995; Watson et al., 1996; Pigorini and LeVan, 1997). For hydrogen purification
using layered activated carbon and zeolite, Chlendi and Tondeur (1995) used the
7
6 5
Amount adsorbed (m mol/g) 4 3 CO 2
CH
4
2
CO
H
2
Langmuir
1 LRC
0
0 2000 4000 6000 8000 10000
Pressure (mmHg)
◦
Figure 10.21. Equilibrium isotherms on activated carbon (Calgon BPL) at 25 C (Park et al.,
1998, with permission).