Page 316 - Adsorbents fundamentals and applications
P. 316
AIR SEPARATION 301
0.12
Adsorption on Co(fluomine)-MCM-41 at 25°C
O
Desorption Adsorption 2
Amount adsorbed, m mol/g 0.10
0.08
0.06
0.04
0.02 N 2
0.00
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Pressure, atm
◦
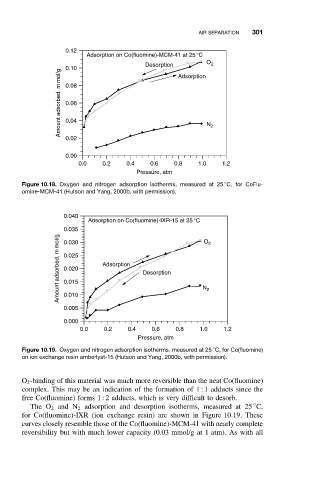
Figure 10.18. Oxygen and nitrogen adsorption isotherms, measured at 25 C, for CoFlu-
omine-MCM-41 (Hutson and Yang, 2000b, with permission).
0.040
Adsorption on Co(fluomine)-IXR-15 at 25°C
0.035 O 2
Amount adsorbed, m mol/g 0.025 Adsorption Desorption N
0.030
0.020
0.015
0.010
0.005 2
0.000
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Pressure, atm
◦
Figure 10.19. Oxygen and nitrogen adsorption isotherms, measured at 25 C, for Co(fluomine)
on ion exchange resin amberlyst-15 (Hutson and Yang, 2000b, with permission).
O 2 -binding of this material was much more reversible than the neat Co(fluomine)
complex. This may be an indication of the formation of 1 : 1 adducts since the
free Co(fluomine) forms 1 : 2 adducts, which is very difficult to desorb.
◦
The O 2 and N 2 adsorption and desorption isotherms, measured at 25 C,
for Co(fluomine)-IXR (ion exchange resin) are shown in Figure 10.19. These
curves closely resemble those of the Co(fluomine)-MCM-41 with nearly complete
reversibility but with much lower capacity (0.03 mmol/g at 1 atm). As with all