Page 304 - Adsorbents fundamentals and applications
P. 304
AIR SEPARATION 289
)
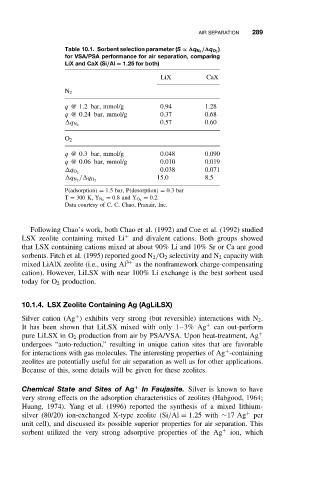
Table 10.1. Sorbent selection parameter (S ∝ q N 2 / q O 2
for VSA/PSA performance for air separation, comparing
LiX and CaX (Si/Al = 1.25 for both)
LiX CaX
N 2
q @ 1.2 bar, mmol/g 0.94 1.28
q @ 0.24 bar, mmol/g 0.37 0.68
0.57 0.60
q N 2
O 2
q @ 0.3 bar, mmol/g 0.048 0.090
q @ 0.06 bar, mmol/g 0.010 0.019
0.038 0.071
q O 2
15.0 8.5
q N 2 / q O 2
P(adsorption) = 1.5bar,P(desorption) = 0.3bar
= 0.2.
T = 300 K, Y N 2 = 0.8and Y O 2
Data courtesy of C. C. Chao, Praxair, Inc.
Following Chao’s work, both Chao et al. (1992) and Coe et al. (1992) studied
LSX zeolite containing mixed Li + and divalent cations. Both groups showed
that LSX containing cations mixed at about 90% Li and 10% Sr or Ca are good
sorbents. Fitch et al. (1995) reported good N 2 /O 2 selectivity and N 2 capacity with
mixed LiAlX zeolite (i.e., using Al 3+ as the nonframework charge-compensating
cation). However, LiLSX with near 100% Li exchange is the best sorbent used
today for O 2 production.
10.1.4. LSX Zeolite Containing Ag (AgLiLSX)
+
Silver cation (Ag ) exhibits very strong (but reversible) interactions with N 2 .
It has been shown that LiLSX mixed with only 1–3% Ag + can out-perform
pure LiLSX in O 2 production from air by PSA/VSA. Upon heat-treatment, Ag +
undergoes “auto-reduction,” resulting in unique cation sites that are favorable
for interactions with gas molecules. The interesting properties of Ag -containing
+
zeolites are potentially useful for air separation as well as for other applications.
Because of this, some details will be given for these zeolites.
Chemical State and Sites of Ag + In Faujasite. Silver is known to have
very strong effects on the adsorption characteristics of zeolites (Habgood, 1964;
Huang, 1974). Yang et al. (1996) reported the synthesis of a mixed lithium-
silver (80/20) ion-exchanged X-type zeolite (Si/Al = 1.25 with ∼17 Ag + per
unit cell), and discussed its possible superior properties for air separation. This
sorbent utilized the very strong adsorptive properties of the Ag + ion, which