Page 300 - Adsorbents fundamentals and applications
P. 300
AIR SEPARATION 285
reached for increased N 2 adsorption, beyond which the amount of N 2 increased
linearly with Li content. Moreover, the O 2 capacity was decreased because the
+
+
+
polarizability of Li is lower than that of Na . Since Chao’s invention, LiLSX
has been the sorbent of choice for air separation.
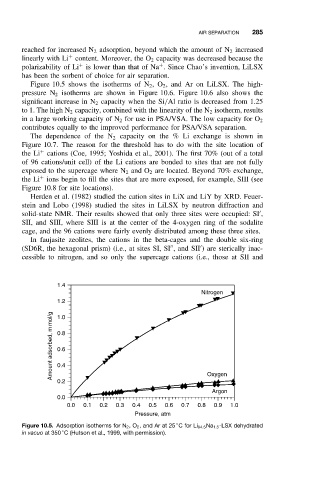
Figure 10.5 shows the isotherms of N 2 ,O 2 , and Ar on LiLSX. The high-
pressure N 2 isotherms are shown in Figure 10.6. Figure 10.6 also shows the
significant increase in N 2 capacity when the Si/Al ratio is decreased from 1.25
to 1. The high N 2 capacity, combined with the linearity of the N 2 isotherm, results
in a large working capacity of N 2 for use in PSA/VSA. The low capacity for O 2
contributes equally to the improved performance for PSA/VSA separation.
The dependence of the N 2 capacity on the % Li exchange is shown in
Figure 10.7. The reason for the threshold has to do with the site location of
+
the Li cations (Coe, 1995; Yoshida et al., 2001). The first 70% (out of a total
of 96 cations/unit cell) of the Li cations are bonded to sites that are not fully
exposed to the supercage where N 2 and O 2 are located. Beyond 70% exchange,
the Li ions begin to fill the sites that are more exposed, for example, SIII (see
+
Figure 10.8 for site locations).
Herden et al. (1982) studied the cation sites in LiX and LiY by XRD. Feuer-
stein and Lobo (1998) studied the sites in LiLSX by neutron diffraction and
solid-state NMR. Their results showed that only three sites were occupied: SI ,
SII, and SIII, where SIII is at the center of the 4-oxygen ring of the sodalite
cage, and the 96 cations were fairly evenly distributed among these three sites.
In faujasite zeolites, the cations in the beta-cages and the double six-ring
(SD6R, the hexagonal prism) (i.e., at sites SI, SI ,and SII ) are sterically inac-
cessible to nitrogen, and so only the supercage cations (i.e., those at SII and
1.4
Nitrogen
1.2
Amount adsorbed, m mol/g 0.8
1.0
0.6
0.4
0.2 Oxygen
Argon
0.0
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Pressure, atm
◦
Figure 10.5. Adsorption isotherms for N 2 ,O 2 , and Ar at 25 Cfor Li 94.5 Na 1.5 -LSX dehydrated
◦
in vacuo at 350 C (Hutson et al., 1999, with permission).