Page 302 - Adsorbents fundamentals and applications
P. 302
AIR SEPARATION 287
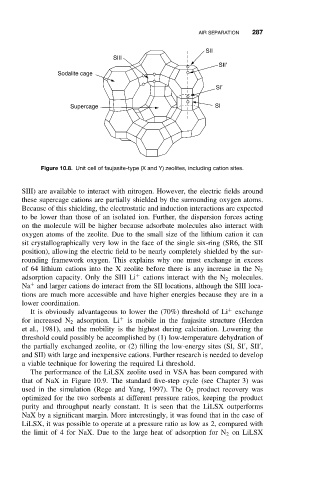
SII
SIII
SII’
Sodalite cage
SI’
Supercage SI
Figure 10.8. Unit cell of faujasite-type (X and Y) zeolites, including cation sites.
SIII) are available to interact with nitrogen. However, the electric fields around
these supercage cations are partially shielded by the surrounding oxygen atoms.
Because of this shielding, the electrostatic and induction interactions are expected
to be lower than those of an isolated ion. Further, the dispersion forces acting
on the molecule will be higher because adsorbate molecules also interact with
oxygen atoms of the zeolite. Due to the small size of the lithium cation it can
sit crystallographically very low in the face of the single six-ring (SR6, the SII
position), allowing the electric field to be nearly completely shielded by the sur-
rounding framework oxygen. This explains why one must exchange in excess
of 64 lithium cations into the X zeolite before there is any increase in the N 2
adsorption capacity. Only the SIII Li cations interact with the N 2 molecules.
+
Na and larger cations do interact from the SII locations, although the SIII loca-
+
tions are much more accessible and have higher energies because they are in a
lower coordination.
+
It is obviously advantageous to lower the (70%) threshold of Li exchange
for increased N 2 adsorption. Li + is mobile in the faujasite structure (Herden
et al., 1981), and the mobility is the highest during calcination. Lowering the
threshold could possibly be accomplished by (1) low-temperature dehydration of
the partially exchanged zeolite, or (2) filling the low-energy sites (SI, SI ,SII ,
and SII) with large and inexpensive cations. Further research is needed to develop
a viable technique for lowering the required Li threshold.
The performance of the LiLSX zeolite used in VSA has been compared with
that of NaX in Figure 10.9. The standard five-step cycle (see Chapter 3) was
used in the simulation (Rege and Yang, 1997). The O 2 product recovery was
optimized for the two sorbents at different pressure ratios, keeping the product
purity and throughput nearly constant. It is seen that the LiLSX outperforms
NaX by a significant margin. More interestingly, it was found that in the case of
LiLSX, it was possible to operate at a pressure ratio as low as 2, compared with
the limit of 4 for NaX. Due to the large heat of adsorption for N 2 on LiLSX