Page 360 - Adsorbents fundamentals and applications
P. 360
DESULFURIZATION OF TRANSPORTATION FUELS 345
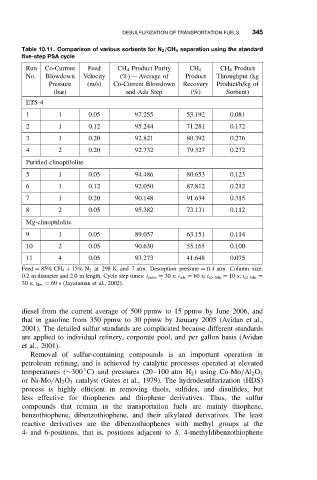
Table 10.11. Comparison of various sorbents for N 2 /CH 4 separation using the standard
five-step PSA cycle
Run Co-Current Feed CH 4 Product Purity CH 4 CH 4 Product
No. Blowdown Velocity (%) — Average of Product Throughput (kg
Pressure (m/s) Co-Current Blowdown Recovery Product/h/kg of
(bar) and Ads Step (%) Sorbent)
ETS-4
1 1 0.05 97.255 53.192 0.081
2 1 0.12 95.244 71.281 0.172
3 1 0.20 92.821 80.392 0.276
4 2 0.20 92.732 79.327 0.272
Purified clinoptilolite
5 1 0.05 94.486 80.653 0.123
6 1 0.12 92.050 87.812 0.212
7 1 0.20 90.148 91.634 0.315
8 2 0.05 95.382 73.131 0.112
Mg-clinoptilolite
9 1 0.05 89.057 63.151 0.114
10 2 0.05 90.630 55.165 0.100
11 4 0.05 93.273 41.648 0.075
Feed = 85% CH 4 + 15% N 2 at 298 K and 7 atm. Desorption pressure = 0.4 atm. Column size:
0.2 m diameter and 2.0 m length. Cycle step times: t press = 30 s; t ads = 60 s; t co bdn = 10 s; t cn bdn =
30 s; t des = 60 s (Jayaraman et al., 2002).
diesel from the current average of 500 ppmw to 15 ppmw by June 2006, and
that in gasoline from 350 ppmw to 30 ppmw by January 2005 (Avidan et al.,
2001). The detailed sulfur standards are complicated because different standards
are applied to individual refinery, corporate pool, and per gallon basis (Avidan
et al., 2001).
Removal of sulfur-containing compounds is an important operation in
petroleum refining, and is achieved by catalytic processes operated at elevated
◦
temperatures (∼300 C) and pressures (20–100 atm H 2 )using Co-Mo/Al 2 O 3
or Ni-Mo/Al 2 O 3 catalyst (Gates et al., 1979). The hydrodesulfurization (HDS)
process is highly efficient in removing thiols, sulfides, and disulfides, but
less effective for thiophenes and thiophene derivatives. Thus, the sulfur
compounds that remain in the transportation fuels are mainly thiophene,
benzothiophene, dibenzothiophene, and their alkylated derivatives. The least
reactive derivatives are the dibenzothiophenes with methyl groups at the
4- and 6-positions, that is, positions adjacent to S. 4-methyldibenzothiophene