Page 357 - Adsorbents fundamentals and applications
P. 357
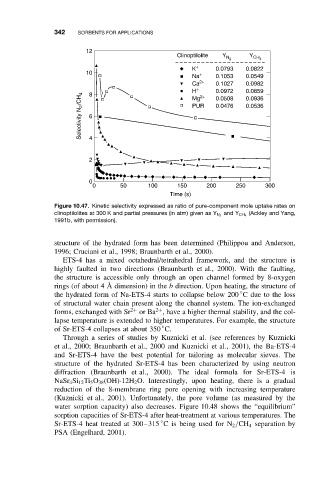
342 SORBENTS FOR APPLICATIONS
12
Clinoptilolite Y N 2 Y CH 4
+
K 0.0793 0.0822
10 +
Na 0.1053 0.0549
Ca 2+ 0.1027 0.0982
+
H 2+ 0.0972 0.0859
8
Selectivity N 2 /CH 4 6 PUR 0.0476 0.0536
0.0508
0.0936
Mg
4
2
0
0 50 100 150 200 250 300
Time (s)
Figure 10.47. Kinetic selectivity expressed as ratio of pure-component mole uptake rates on
and Y CH 4 (Ackley and Yang,
clinoptilolites at 300 K and partial pressures (in atm) given as Y N 2
1991b, with permission).
structure of the hydrated form has been determined (Philippou and Anderson,
1996; Cruciani et al., 1998; Braunbarth et al., 2000).
ETS-4 has a mixed octahedral/tetrahedral framework, and the structure is
highly faulted in two directions (Braunbarth et al., 2000). With the faulting,
the structure is accessible only through an open channel formed by 8-oxygen
rings (of about 4 ˚ A dimension) in the b direction. Upon heating, the structure of
◦
the hydrated form of Na-ETS-4 starts to collapse below 200 C due to the loss
of structural water chain present along the channel system. The ion-exchanged
2+
forms, exchanged with Sr 2+ or Ba , have a higher thermal stability, and the col-
lapse temperature is extended to higher temperatures. For example, the structure
◦
of Sr-ETS-4 collapses at about 350 C.
Through a series of studies by Kuznicki et al. (see references by Kuznicki
et al., 2000; Braunbarth et al., 2000 and Kuznicki et al., 2001), the Ba-ETS-4
and Sr-ETS-4 have the best potential for tailoring as molecular sieves. The
structure of the hydrated Sr-ETS-4 has been characterized by using neutron
diffraction (Braunbarth et al., 2000). The ideal formula for Sr-ETS-4 is
NaSr 4 Si 12 Ti 5 O 38 (OH)·12H 2 O. Interestingly, upon heating, there is a gradual
reduction of the 8-membrane ring pore opening with increasing temperature
(Kuznicki et al., 2001). Unfortunately, the pore volume (as measured by the
water sorption capacity) also decreases. Figure 10.48 shows the “equilibrium”
sorption capacities of Sr-ETS-4 after heat-treatment at various temperatures. The
◦
Sr-ETS-4 heat treated at 300–315 C is being used for N 2 /CH 4 separation by
PSA (Engelhard, 2001).