Page 354 - Adsorbents fundamentals and applications
P. 354
NITROGEN/METHANE SEPARATION 339
5
4
Selectivity ratio CH 4 /N 2 3 H + + 2+
K
2
Mg
1 PUR
+
Na
Ca 2+
0
0.0 0.2 0.4 0.6 0.8 1.0
Partial pressure atm
Figure 10.42. Pure-component equilibrium selectivity ratio of CH 4 /N 2 adsorption on ion-ex-
changed clinoptilolites at 300 K (Ackley and Yang, 1991a, with permission). PUR: purified form.
1.2 Nitrogen
Amount adsorbed (m mol/g) 0.8 Purified clinoptilolite
1
Methane
0.6
0.4
0.2
0
0 1 2 3 4 5 6 7
Pressure (atm)
◦
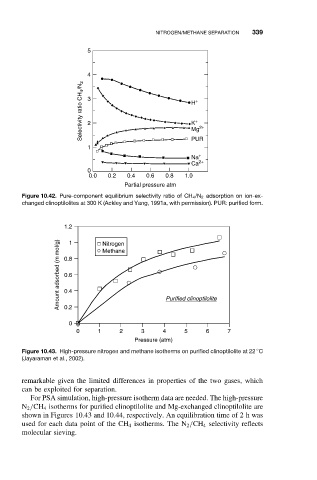
Figure 10.43. High-pressure nitrogen and methane isotherms on purified clinoptilolite at 22 C
(Jayaraman et al., 2002).
remarkable given the limited differences in properties of the two gases, which
can be exploited for separation.
For PSA simulation, high-pressure isotherm data are needed. The high-pressure
N 2 /CH 4 isotherms for purified clinoptilolite and Mg-exchanged clinoptilolite are
shown in Figures 10.43 and 10.44, respectively. An equilibration time of 2 h was
used for each data point of the CH 4 isotherms. The N 2 /CH 4 selectivity reflects
molecular sieving.