Page 351 - Adsorbents fundamentals and applications
P. 351
336 SORBENTS FOR APPLICATIONS
2.0
293 K Closed symbol: propylene
303 K Open symbol: propane
333 K
363 K
313 K
A
1.5
m mol/g 1.0
B
0.5
0.0
0 200 400 600 800 1000 1200
p, mmHg
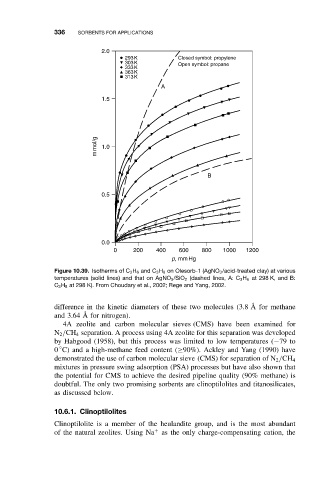
Figure 10.39. Isotherms of C 3 H 6 and C 3 H 8 on Olesorb-1 (AgNO 3 /acid-treated clay) at various
temperatures (solid lines) and that on AgNO 3 /SiO 2 (dashed lines, A: C 3 H 6 at 298 K, and B:
C 3 H 8 at 298 K). From Choudary et al., 2002; Rege and Yang, 2002.
difference in the kinetic diameters of these two molecules (3.8 ˚ Afor methane
and 3.64 ˚ A for nitrogen).
4A zeolite and carbon molecular sieves (CMS) have been examined for
N 2 /CH 4 separation. A process using 4A zeolite for this separation was developed
by Habgood (1958), but this process was limited to low temperatures (−79 to
◦
0 C) and a high-methane feed content (≥90%). Ackley and Yang (1990) have
demonstrated the use of carbon molecular sieve (CMS) for separation of N 2 /CH 4
mixtures in pressure swing adsorption (PSA) processes but have also shown that
the potential for CMS to achieve the desired pipeline quality (90% methane) is
doubtful. The only two promising sorbents are clinoptilolites and titanosilicates,
as discussed below.
10.6.1. Clinoptilolites
Clinoptilolite is a member of the heulandite group, and is the most abundant
of the natural zeolites. Using Na as the only charge-compensating cation, the
+