Page 353 - Adsorbents fundamentals and applications
P. 353
338 SORBENTS FOR APPLICATIONS
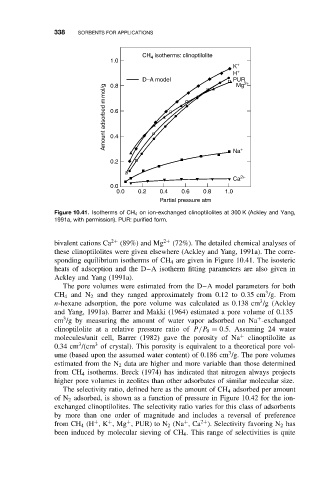
CH isotherms: clinoptilolite
4
1.0
K +
H +
D–A model PUR 2+
Mg
0.8
Amount adsorbed m mol/g 0.6
0.4
Na +
0.2
Ca 2+
0.0
0.0 0.2 0.4 0.6 0.8 1.0
Partial pressure atm
Figure 10.41. Isotherms of CH 4 on ion-exchanged clinoptilolites at 300 K (Ackley and Yang,
1991a, with permission). PUR: purified form.
bivalent cations Ca 2+ (89%) and Mg 2+ (72%). The detailed chemical analyses of
these clinoptilolites were given elsewhere (Ackley and Yang, 1991a). The corre-
sponding equilibrium isotherms of CH 4 are given in Figure 10.41. The isosteric
heats of adsorption and the D–A isotherm fitting parameters are also given in
Ackley and Yang (1991a).
The pore volumes were estimated from the D–A model parameters for both
3
CH 4 and N 2 and they ranged approximately from 0.12 to 0.35 cm /g. From
3
n-hexane adsorption, the pore volume was calculated as 0.138 cm /g (Ackley
and Yang, 1991a). Barrer and Makki (1964) estimated a pore volume of 0.135
3
+
cm /g by measuring the amount of water vapor adsorbed on Na -exchanged
clinoptilolite at a relative pressure ratio of P/P 0 = 0.5. Assuming 24 water
molecules/unit cell, Barrer (1982) gave the porosity of Na + clinoptilolite as
3
3
0.34 cm /(cm of crystal). This porosity is equivalent to a theoretical pore vol-
3
ume (based upon the assumed water content) of 0.186 cm /g. The pore volumes
estimated from the N 2 data are higher and more variable than those determined
from CH 4 isotherms. Breck (1974) has indicated that nitrogen always projects
higher pore volumes in zeolites than other adsorbates of similar molecular size.
The selectivity ratio, defined here as the amount of CH 4 adsorbed per amount
of N 2 adsorbed, is shown as a function of pressure in Figure 10.42 for the ion-
exchanged clinoptilolites. The selectivity ratio varies for this class of adsorbents
by more than one order of magnitude and includes a reversal of preference
+
+
+
+
2+
from CH 4 (H ,K ,Mg ,PUR) toN 2 (Na ,Ca ). Selectivity favoring N 2 has
been induced by molecular sieving of CH 4 . This range of selectivities is quite