Page 366 - Adsorbents fundamentals and applications
P. 366
DESULFURIZATION OF TRANSPORTATION FUELS 351
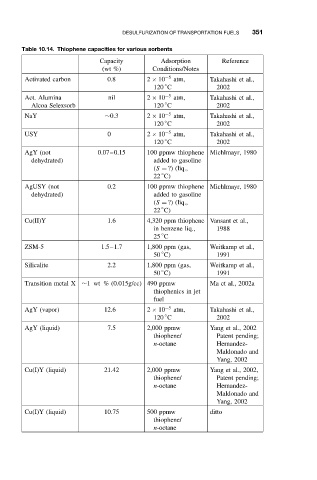
Table 10.14. Thiophene capacities for various sorbents
Capacity Adsorption Reference
(wt %) Conditions/Notes
Activated carbon 0.8 2 × 10 −5 atm, Takahashi et al.,
◦
120 C 2002
Act. Alumina nil 2 × 10 −5 atm, Takahashi et al.,
◦
Alcoa Selexsorb 120 C 2002
NaY ∼0.3 2 × 10 −5 atm, Takahashi et al.,
◦
120 C 2002
USY 0 2 × 10 −5 atm, Takahashi et al.,
◦
120 C 2002
AgY (not 0.07–0.15 100 ppmw thiophene Michlmayr, 1980
dehydrated) addedtogasoline
(S = ?) (liq.,
◦
22 C)
AgUSY (not 0.2 100 ppmw thiophene Michlmayr, 1980
dehydrated) addedtogasoline
(S = ?) (liq.,
◦
22 C)
Cu(II)Y 1.6 4,320 ppm thiophene Vansant et al.,
in benzene liq., 1988
◦
25 C
ZSM-5 1.5–1.7 1,800 ppm (gas, Weitkamp et al.,
◦
50 C) 1991
Silicalite 2.2 1,800 ppm (gas, Weitkamp et al.,
◦
50 C) 1991
Transition metal X ∼1 wt % (0.015g/cc) 490 ppmw Ma et al., 2002a
thiophenics in jet
fuel
AgY (vapor) 12.6 2 × 10 −5 atm, Takahashi et al.,
◦
120 C 2002
AgY (liquid) 7.5 2,000 ppmw Yang et al., 2002
thiophene/ Patent pending;
n-octane Hernandez-
Maldonado and
Yang, 2002
Cu(I)Y (liquid) 21.42 2,000 ppmw Yang et al., 2002,
thiophene/ Patent pending;
n-octane Hernandez-
Maldonado and
Yang, 2002
Cu(I)Y (liquid) 10.75 500 ppmw ditto
thiophene/
n-octane