Page 367 - Adsorbents fundamentals and applications
P. 367
352 SORBENTS FOR APPLICATIONS
1.2
Relative JP-8 before treatment
sulfur 1.0
conc.
0.8
0.6
0.4
0.2
0.0
0 10 20 30 40 50 60 70 80
Elution volume (ml)
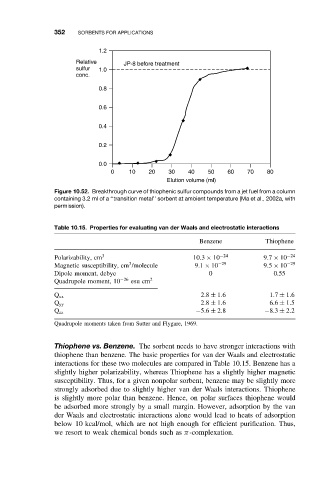
Figure 10.52. Breakthrough curve of thiophenic sulfur compounds from a jet fuel from a column
containing 3.2 ml of a ‘‘transition metal’’ sorbent at ambient temperature (Ma et al., 2002a, with
permission).
Table 10.15. Properties for evaluating van der Waals and electrostatic interactions
Benzene Thiophene
Polarizability, cm 3 10.3 × 10 −24 9.7 × 10 −24
3
Magnetic susceptibility, cm /molecule 9.1 × 10 −29 9.5 × 10 −29
Dipole moment, debye 0 0.55
Quadrupole moment, 10 −26 esu cm 2
Q xx 2.8 ± 1.6 1.7 ± 1.6
Q yy 2.8 ± 1.6 6.6 ± 1.5
Q zz −5.6 ± 2.8 −8.3 ± 2.2
Quadrupole moments taken from Sutter and Flygare, 1969.
Thiophene vs. Benzene. The sorbent needs to have stronger interactions with
thiophene than benzene. The basic properties for van der Waals and electrostatic
interactions for these two molecules are compared in Table 10.15. Benzene has a
slightly higher polarizability, whereas Thiophene has a slightly higher magnetic
susceptibility. Thus, for a given nonpolar sorbent, benzene may be slightly more
strongly adsorbed due to slightly higher van der Waals interactions. Thiophene
is slightly more polar than benzene. Hence, on polar surfaces thiophene would
be adsorbed more strongly by a small margin. However, adsorption by the van
der Waals and electrostatic interactions alone would lead to heats of adsorption
below 10 kcal/mol, which are not high enough for efficient purification. Thus,
we resort to weak chemical bonds such as π-complexation.