Page 372 - Adsorbents fundamentals and applications
P. 372
DESULFURIZATION OF TRANSPORTATION FUELS 357
at 3 × 10 −5 atm and 2 × 10 −2 atm was preferable for the purification of ben-
zene by removal of thiophene. A disadvantage of Na-ZSM-5 was that the small
adsorption capacity was limited by the small pore volume of ZSM-5. Moreover,
no studies have been reported on the adsorption of benzothiophene, dibenzoth-
iophene, and their derivatives on ZSM-5. The pore dimensions of ZSM-5 are
5.2 × 5.6 ˚ A. These sulfur compounds (with more than one ring) will likely be
sterically hindered or excluded by ZSM-5.
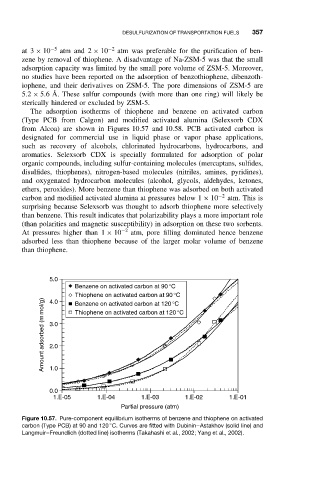
The adsorption isotherms of thiophene and benzene on activated carbon
(Type PCB from Calgon) and modified activated alumina (Selexsorb CDX
from Alcoa) are shown in Figures 10.57 and 10.58. PCB activated carbon is
designated for commercial use in liquid phase or vapor phase applications,
such as recovery of alcohols, chlorinated hydrocarbons, hydrocarbons, and
aromatics. Selexsorb CDX is specially formulated for adsorption of polar
organic compounds, including sulfur-containing molecules (mercaptans, sulfides,
disulfides, thiophenes), nitrogen-based molecules (nitriles, amines, pyridines),
and oxygenated hydrocarbon molecules (alcohol, glycols, aldehydes, ketones,
ethers, peroxides). More benzene than thiophene was adsorbed on both activated
carbon and modified activated alumina at pressures below 1 × 10 −2 atm. This is
surprising because Selexsorb was thought to adsorb thiophene more selectively
than benzene. This result indicates that polarizability plays a more important role
(than polarities and magnetic susceptibility) in adsorption on these two sorbents.
At pressures higher than 1 × 10 −2 atm, pore filling dominated hence benzene
adsorbed less than thiophene because of the larger molar volume of benzene
than thiophene.
5.0
Benzene on activated carbon at 90 °C
Thiophene on activated carbon at 90 °C
Amount adsorbed (m mol/g) 3.0
4.0
Benzene on activated carbon at 120 °C
Thiophene on activated carbon at 120 °C
2.0
1.0
0.0
1.E-05 1.E-04 1.E-03 1.E-02 1.E-01
Partial pressure (atm)
Figure 10.57. Pure-component equilibrium isotherms of benzene and thiophene on activated
◦
carbon (Type PCB) at 90 and 120 C. Curves are fitted with Dubinin–Astakhov (solid line) and
Langmuir–Freundlich (dotted line) isotherms (Takahashi et al., 2002; Yang et al., 2002).