Page 370 - Adsorbents fundamentals and applications
P. 370
DESULFURIZATION OF TRANSPORTATION FUELS 355
2.5
Amount adsorbed (m mol/g) 1.5
2.0
1.0
Benzene on Ag-Y at 120°C
0.5
Thiophene on Ag-Y at 120°C
Benzene on Ag-Y at 180°C
Thiophene on Ag-Y at 180°C
0.0
1.E-06 1.E-05 1.E-04 1.E-03 1.E-02 1.E-01
Partial pressure (atm)
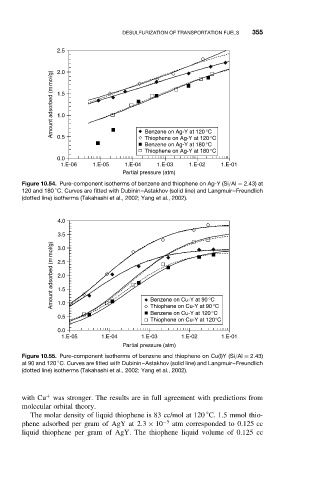
Figure 10.54. Pure-component isotherms of benzene and thiophene on Ag-Y (Si/Al = 2.43) at
◦
120 and 180 C. Curves are fitted with Dubinin–Astakhov (solid line) and Langmuir–Freundlich
(dotted line) isotherms (Takahashi et al., 2002; Yang et al., 2002).
4.0
3.5
Amount adsorbed (m mol/g) 2.5
3.0
2.0
1.5
Benzene on Cu-Y at 90 °C
1.0
Thiophene on Cu-Y at 90°C
Benzene on Cu-Y at 120°C
0.5
Thiophene on Cu-Y at 120°C
0.0
1.E-05 1.E-04 1.E-03 1.E-02 1.E-01
Partial pressure (atm)
Figure 10.55. Pure-component isotherms of benzene and thiophene on Cu(I)Y (Si/Al = 2.43)
at 90 and 120 C. Curves are fitted with Dubinin–Astakhov (solid line) and Langmuir–Freundlich
◦
(dotted line) isotherms (Takahashi et al., 2002; Yang et al., 2002).
+
with Cu was stronger. The results are in full agreement with predictions from
molecular orbital theory.
◦
The molar density of liquid thiophene is 83 cc/mol at 120 C. 1.5 mmol thio-
phene adsorbed per gram of AgY at 2.3 × 10 −5 atm corresponded to 0.125 cc
liquid thiophene per gram of AgY. The thiophene liquid volume of 0.125 cc