Page 369 - Adsorbents fundamentals and applications
P. 369
354 SORBENTS FOR APPLICATIONS
3.5
Benzene on Na-Y at 120°C
Thiophene on Na-Y at 120°C
3.0
Benzene on Na-Y at 180°C
Amount adsorbed (m mol/g) 2.0
Thiophene on Na-Y at 180°C
2.5
1.5
1.0
0.5
0.0
1.E-05 1.E-04 1.E-03 1.E-02 1.E-01
Partial pressure (atm)
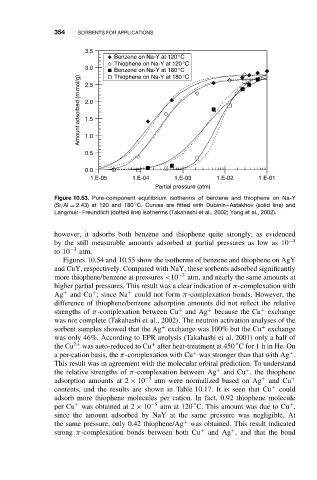
Figure 10.53. Pure-component equilibrium isotherms of benzene and thiophene on Na-Y
◦
(Si/Al = 2.43) at 120 and 180 C. Curves are fitted with Dubinin–Astakhov (solid line) and
Langmuir–Freundlich (dotted line) isotherms (Takahashi et al., 2002; Yang et al., 2002).
however, it adsorbs both benzene and thiophene quite strongly, as evidenced
by the still measurable amounts adsorbed at partial pressures as low as 10 −4
to 10 −3 atm.
Figures 10.54 and 10.55 show the isotherms of benzene and thiophene on AgY
and CuY, respectively. Compared with NaY, these sorbents adsorbed significantly
more thiophene/benzene at pressures <10 −3 atm, and nearly the same amounts at
higher partial pressures. This result was a clear indication of π-complexation with
+
+
Ag and Cu ;since Na could not form π-complexation bonds. However, the
+
difference of thiophene/benzene adsorption amounts did not reflect the relative
+
+
strengths of π-complexation between Cu and Ag because the Cu exchange
+
was not complete (Takahashi et al., 2002). The neutron activation analyses of the
sorbent samples showed that the Ag exchange was 100% but the Cu exchange
+
+
was only 46%. According to EPR analysis (Takahashi et al, 2001) only a half of
◦
+
the Cu 2+ was auto-reduced to Cu after heat-treatment at 450 Cfor 1 h in He. On
+
+
a per-cation basis, the π-complexation with Cu was stronger than that with Ag .
This result was in agreement with the molecular orbital prediction. To understand
+
the relative strengths of π-complexation between Ag and Cu , the thiophene
+
adsorption amounts at 2 × 10 −5 atm were normalized based on Ag + and Cu +
contents, and the results are shown in Table 10.17. It is seen that Cu + could
adsorb more thiophene molecules per cation. In fact, 0.92 thiophene molecule
◦
per Cu was obtained at 2 × 10 −5 atm at 120 C. This amount was due to Cu ,
+
+
since the amount adsorbed by NaY at the same pressure was negligible. At
the same pressure, only 0.42 thiophene/Ag was obtained. This result indicated
+
+
strong π-complexation bonds between both Cu + and Ag , and that the bond