Page 373 - Adsorbents fundamentals and applications
P. 373
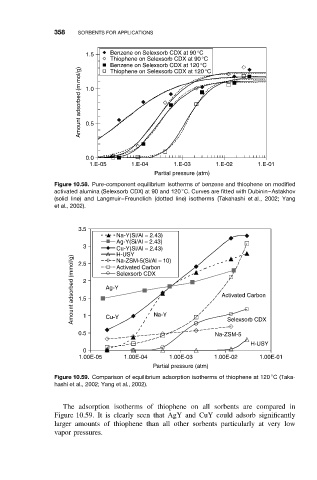
358 SORBENTS FOR APPLICATIONS
1.5 Benzene on Selexsorb CDX at 90°C
Thiophene on Selexsorb CDX at 90°C
Benzene on Selexsorb CDX at 120°C
Amount adsorbed (m mol/g) 1.0
Thiophene on Selexsorb CDX at 120°C
0.5
0.0
1.E-05 1.E-04 1.E-03 1.E-02 1.E-01
Partial pressure (atm)
Figure 10.58. Pure-component equilibrium isotherms of benzene and thiophene on modified
◦
activated alumina (Selexsorb CDX) at 90 and 120 C. Curves are fitted with Dubinin–Astakhov
(solid line) and Langmuir–Freundlich (dotted line) isotherms (Takahashi et al., 2002; Yang
et al., 2002).
3.5
Na-Y(Si/Al = 2.43)
Ag-Y(Si/Al = 2.43)
3 Cu-Y(Si/Al = 2.43)
H-USY
Amount adsorbed (mmol/g) 1.5 2 Ag-Y Selexsorb CDX Activated Carbon
Na-ZSM-5(Si/Al = 10)
2.5
Activated Carbon
Selexsorb CDX
0.5 1 Cu-Y Na-Y Na-ZSM-5
H-USY
0
1.00E-05 1.00E-04 1.00E-03 1.00E-02 1.00E-01
Partial pressure (atm)
◦
Figure 10.59. Comparison of equilibrium adsorption isotherms of thiophene at 120 C(Taka-
hashi et al., 2002; Yang et al., 2002).
The adsorption isotherms of thiophene on all sorbents are compared in
Figure 10.59. It is clearly seen that AgY and CuY could adsorb significantly
larger amounts of thiophene than all other sorbents particularly at very low
vapor pressures.