Page 377 - Adsorbents fundamentals and applications
P. 377
362 SORBENTS FOR APPLICATIONS
2.5
Benzene (120°C)
Benzene (180°C)
Cyclohexane (120°C)
2.0
Cyclohexane (180°C)
q (m mol/g) 1.5
1.0
0.5
0.0
1.E−6 1.E−5 1.E−4 1.E−3 1.E−2 1.E−1 1.E+0
Partial pressure (atm)
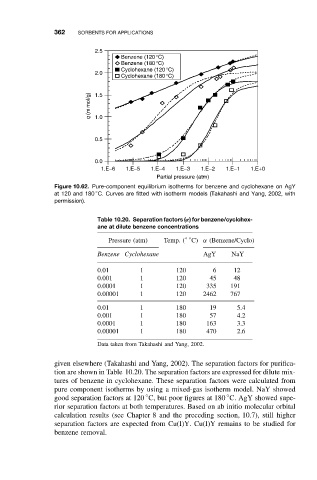
Figure 10.62. Pure-component equilibrium isotherms for benzene and cyclohexane on AgY
◦
at 120 and 180 C. Curves are fitted with isotherm models (Takahashi and Yang, 2002, with
permission).
Table 10.20. Separation factors (α) for benzene/cyclohex-
ane at dilute benzene concentrations
◦◦
Pressure (atm) Temp. ( C) α (Benzene/Cyclo)
Benzene Cyclohexane AgY NaY
0.01 1 120 6 12
0.001 1 120 45 48
0.0001 1 120 335 191
0.00001 1 120 2462 767
0.01 1 180 19 5.4
0.001 1 180 57 4.2
0.0001 1 180 163 3.3
0.00001 1 180 470 2.6
Data taken from Takahashi and Yang, 2002.
given elsewhere (Takahashi and Yang, 2002). The separation factors for purifica-
tion are shown in Table 10.20. The separation factors are expressed for dilute mix-
tures of benzene in cyclohexane. These separation factors were calculated from
pure component isotherms by using a mixed-gas isotherm model. NaY showed
◦
◦
good separation factors at 120 C, but poor figures at 180 C. AgY showed supe-
rior separation factors at both temperatures. Based on ab initio molecular orbital
calculation results (see Chapter 8 and the preceding section, 10.7), still higher
separation factors are expected from Cu(I)Y. Cu(I)Y remains to be studied for
benzene removal.