Page 381 - Adsorbents fundamentals and applications
P. 381
366 SORBENTS FOR APPLICATIONS
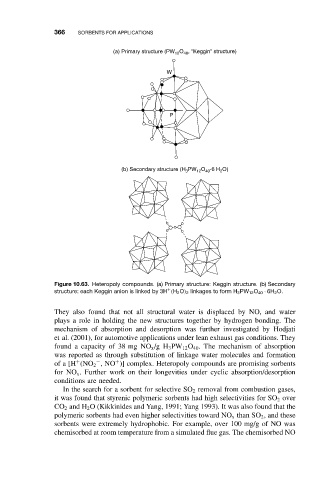
(a) Primary structure (PW 12 O 40 , “Keggin” structure)
W
P
(b) Secondary structure (H 3 PW 12 O 40 ·6 H 2 O)
Figure 10.63. Heteropoly compounds. (a) Primary structure: Keggin structure. (b) Secondary
+
structure: each Keggin anion is linked by 3H (H 2 O) 2 linkages to form H 3 PW 12 O 40 · 6H 2 O.
They also found that not all structural water is displaced by NO, and water
plays a role in holding the new structures together by hydrogen bonding. The
mechanism of absorption and desorption was further investigated by Hodjati
et al. (2001), for automotive applications under lean exhaust gas conditions. They
found a capacity of 38 mg NO x /g H 3 PW 12 O 40 . The mechanism of absorption
was reported as through substitution of linkage water molecules and formation
+
+
of a [H (NO 2 ,NO )] complex. Heteropoly compounds are promising sorbents
−
for NO x . Further work on their longevities under cyclic absorption/desorption
conditions are needed.
In the search for a sorbent for selective SO 2 removal from combustion gases,
it was found that styrenic polymeric sorbents had high selectivities for SO 2 over
CO 2 and H 2 O (Kikkinides and Yang, 1991; Yang 1993). It was also found that the
polymeric sorbents had even higher selectivities toward NO x than SO 2 ,and these
sorbents were extremely hydrophobic. For example, over 100 mg/g of NO was
chemisorbed at room temperature from a simulated flue gas. The chemisorbed NO