Page 382 - Adsorbents fundamentals and applications
P. 382
NO X REMOVAL 367
forms monomers or dimers on the benzene rings of the surface of the polymer,
possibly by π-complex bonding with the π-electrons. However, desorption of
NO requires temperatures near the thermal stability temperatures of the styrenic
◦
polymers, that is near 200 C. Polymeric sorbents with higher thermal stabilities
such as the acrylics types need to be tested for this application.
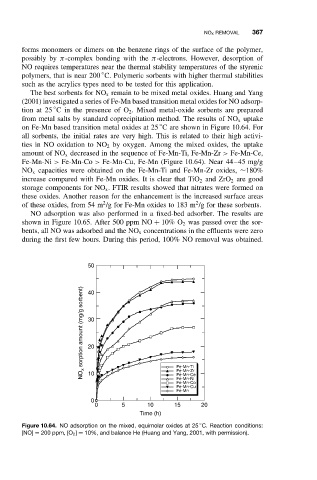
The best sorbents for NO x remain to be mixed metal oxides. Huang and Yang
(2001) investigated a series of Fe-Mn based transition metal oxides for NO adsorp-
◦
tion at 25 C in the presence of O 2 . Mixed metal-oxide sorbents are prepared
from metal salts by standard coprecipitation method. The results of NO x uptake
◦
on Fe-Mn based transition metal oxides at 25 C are shown in Figure 10.64. For
all sorbents, the initial rates are very high. This is related to their high activi-
ties in NO oxidation to NO 2 by oxygen. Among the mixed oxides, the uptake
amount of NO x decreased in the sequence of Fe-Mn-Ti, Fe-Mn-Zr > Fe-Mn-Ce,
Fe-Mn-Ni > Fe-Mn-Co > Fe-Mn-Cu, Fe-Mn (Figure 10.64). Near 44–45 mg/g
NO x capacities were obtained on the Fe-Mn-Ti and Fe-Mn-Zr oxides, ∼180%
increase compared with Fe-Mn oxides. It is clear that TiO 2 and ZrO 2 are good
storage components for NO x . FTIR results showed that nitrates were formed on
these oxides. Another reason for the enhancement is the increased surface areas
2
2
of these oxides, from 54 m /g for Fe-Mn oxides to 183 m /g for these sorbents.
NO adsorption was also performed in a fixed-bed adsorber. The results are
shown in Figure 10.65. After 500 ppm NO + 10% O 2 was passed over the sor-
bents, all NO was adsorbed and the NO x concentrations in the effluents were zero
during the first few hours. During this period, 100% NO removal was obtained.
50
NO x sorption amount (mg/g sorbent) 30
40
20
Fe-Mn-Ti
Fe-Mn-Zr
10
Fe-Mn-Ce
Fe-Mn-Ni
Fe-Mn-Co
Fe-Mn-Cu
Fe-Mn
0
0 5 10 15 20
Time (h)
◦
Figure 10.64. NO adsorption on the mixed, equimolar oxides at 25 C. Reaction conditions:
[NO] = 200 ppm, [O 2 ] = 10%, and balance He (Huang and Yang, 2001, with permission).