Page 383 - Adsorbents fundamentals and applications
P. 383
368 SORBENTS FOR APPLICATIONS
100
Fe-Mn-Ti
Fe-Mn-Zr
Fe-Mn-Ce
80 Fe-Mn-Ni
Fe-Mn-Co
Fe-Mn-Cu
Fe-Mn
NO Removal (%) 40
60
20
0
0 2 4 6 8 10
Time (h)
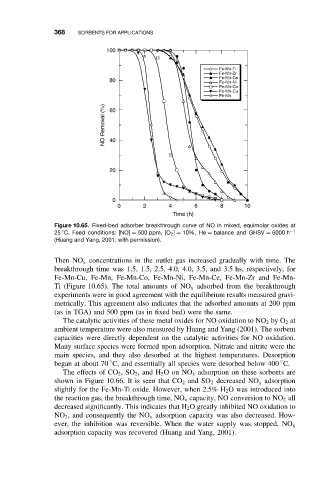
Figure 10.65. Fixed-bed adsorber breakthrough curve of NO in mixed, equimolar oxides at
◦
25 C. Feed conditions: [NO] = 500 ppm, [O 2 ] = 10%, He = balance and GHSV = 6000 h −1
(Huang and Yang, 2001; with permission).
Then NO x concentrations in the outlet gas increased gradually with time. The
breakthrough time was 1.5, 1.5, 2.5, 4.0, 4.0, 3.5, and 3.5 hs, respectively, for
Fe-Mn-Cu, Fe-Mn, Fe-Mn-Co, Fe-Mn-Ni, Fe-Mn-Ce, Fe-Mn-Zr and Fe-Mn-
Ti (Figure 10.65). The total amounts of NO x adsorbed from the breakthrough
experiments were in good agreement with the equilibrium results measured gravi-
metrically. This agreement also indicates that the adsorbed amounts at 200 ppm
(as in TGA) and 500 ppm (as in fixed bed) were the same.
The catalytic activities of these metal oxides for NO oxidation to NO 2 by O 2 at
ambient temperature were also measured by Huang and Yang (2001). The sorbent
capacities were directly dependent on the catalytic activities for NO oxidation.
Many surface species were formed upon adsorption. Nitrate and nitrite were the
main species, and they also desorbed at the highest temperatures. Desorption
◦
◦
began at about 70 C, and essentially all species were desorbed below 400 C.
The effects of CO 2 ,SO 2 ,and H 2 OonNO x adsorption on these sorbents are
shown in Figure 10.66. It is seen that CO 2 and SO 2 decreased NO x adsorption
slightly for the Fe-Mn-Ti oxide. However, when 2.5% H 2 O was introduced into
the reaction gas, the breakthrough time, NO x capacity, NO conversion to NO 2 all
decreased significantly. This indicates that H 2 O greatly inhibited NO oxidation to
NO 2 , and consequently the NO x adsorption capacity was also decreased. How-
ever, the inhibition was reversible. When the water supply was stopped, NO x
adsorption capacity was recovered (Huang and Yang, 2001).