Page 374 - Adsorbents fundamentals and applications
P. 374
DESULFURIZATION OF TRANSPORTATION FUELS 359
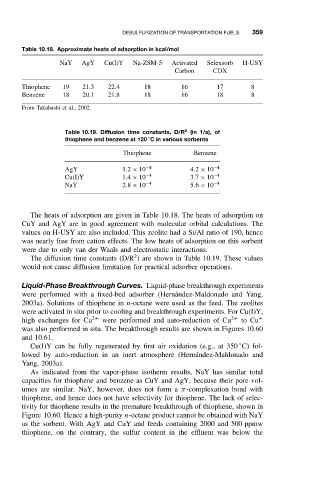
Table 10.18. Approximate heats of adsorption in kcal/mol
NaY AgY Cu(I)Y Na-ZSM-5 Activated Selexsorb H-USY
Carbon CDX
Thiophene 19 21.3 22.4 18 16 17 8
Benzene 18 20.1 21.8 18 16 18 8
From Takahashi et al., 2002.
2
Table 10.19. Diffusion time constants, D/R (in 1/s), of
◦
thiophene and benzene at 120 C in various sorbents
Thiophene Benzene
AgY 1.2 × 10 −4 4.2 × 10 −4
Cu(I)Y 1.4 × 10 −4 3.7 × 10 −4
NaY 2.8 × 10 −4 5.6 × 10 −4
The heats of adsorption are given in Table 10.18. The heats of adsorption on
CuY and AgY are in good agreement with molecular orbital calculations. The
values on H-USY are also included. This zeolite had a Si/Al ratio of 190, hence
was nearly free from cation effects. The low heats of adsorption on this sorbent
were due to only van der Waals and electrostatic interactions.
2
The diffusion time constants (D/R ) are shown in Table 10.19. These values
would not cause diffusion limitation for practical adsorber operations.
Liquid-Phase Breakthrough Curves. Liquid-phase breakthrough experiments
were performed with a fixed-bed adsorber (Hern´ andez-Maldonado and Yang,
2003a). Solutions of thiophene in n-octane were used as the feed. The zeolites
were activated in situ prior to cooling and breakthrough experiments. For Cu(I)Y,
high exchanges for Cu 2+ were performed and auto-reduction of Cu 2+ to Cu +
was also performed in situ. The breakthrough results are shown in Figures 10.60
and 10.61.
◦
Cu(I)Y can be fully regenerated by first air oxidation (e.g., at 350 C) fol-
lowed by auto-reduction in an inert atmosphere (Hern´ andez-Maldonado and
Yang, 2003a).
As indicated from the vapor-phase isotherm results, NaY has similar total
capacities for thiophene and benzene as CuY and AgY, because their pore vol-
umes are similar. NaY, however, does not form a π-complexation bond with
thiophene, and hence does not have selectivity for thiophene. The lack of selec-
tivity for thiophene results in the premature breakthrough of thiophene, shown in
Figure 10.60. Hence a high-purity n-octane product cannot be obtained with NaY
as the sorbent. With AgY and CuY and feeds containing 2000 and 500 ppmw
thiophene, on the contrary, the sulfur content in the effluent was below the