Page 13 - Adsorption Technology & Design, Elsevier (1998)
P. 13
10 Adsorbents
100
a
b
c
r
II)
z.,.
0
r
0
,.,50
C
n
/1
g
,/
\
0J i
0.1 0.5 1 5 10 100 1000
Pore diameter (nm)
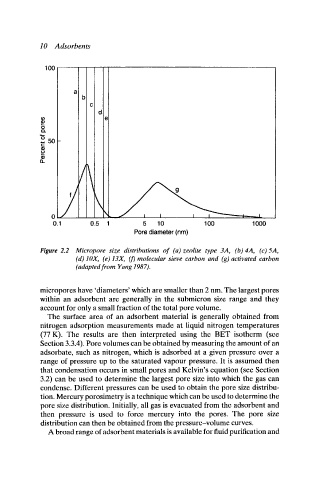
Figure 2.2 Micropore size distributions of (a) zeolite type 3A, (b) 4A, (c) 5A,
(d) IOX, (e)13X, (f) molecular sieve carbon and (g) activated carbon
(adapted from )rang 1987).
micropores have 'diameters' which are smaller than 2 nm. The largest pores
within an adsorbent are generally in the submicron size range and they
account for only a small fraction of the total pore volume.
The surface area of an adsorbent material is generally obtained from
nitrogen adsorption measurements made at liquid nitrogen temperatures
(77 K). The results are then interpreted using the BET isotherm (see
Section 3.3.4). Pore volumes can be obtained by measuring the amount of an
adsorbate, such as nitrogen, which is adsorbed at a given pressure over a
range of pressure up to the saturated vapour pressure. It is assumed then
that condensation occurs in small pores and Kelvin's equation (see Section
3.2) can be used to determine the largest pore size into which the gas can
condense. Different pressures can be used to obtain the pore size distribu-
tion. Mercury porosimetry is a technique which can be used to determine the
pore size distribution. Initially, all gas is evacuated from the adsorbent and
then pressure is used to force mercury into the pores. The pore size
distribution can then be obtained from the pressure-volume curves.
A broad range of adsorbent materials is available for fluid purification and