Page 17 - Adsorption Technology & Design, Elsevier (1998)
P. 17
14 Adsorbents
02
1.0
Fraction
of maximum
(equilibrium)
loading N2
30 60 90
Time minutes.
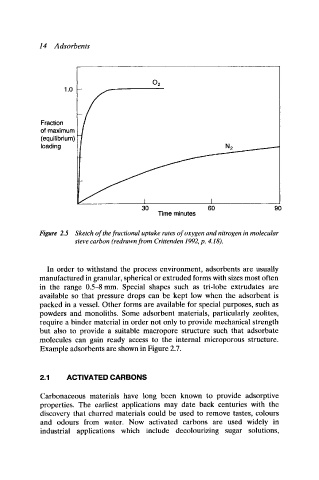
Figure 2.5 Sketch of the fractional uptake rates of oxygen and nitrogen in molecular
sieve carbon (redrawn from Crittenden 1992, p. 4.18).
In order to withstand the process environment, adsorbents are usually
manufactured in granular, spherical or extruded forms with sizes most often
in the range 0.5-8 mm. Special shapes such as tri-lobe extrudates are
available so that pressure drops can be kept low when the adsorbent is
packed in a vessel. Other forms are available for special purposes, such as
powders and monoliths. Some adsorbent materials, particularly zeolites,
require a binder material in order not only to provide mechanical strength
but also to provide a suitable macropore structure such that adsorbate
molecules can gain ready access to the internal microporous structure.
Example adsorbents are shown in Figure 2.7.
2.1 ACTIVATED CARBONS
Carbonaceous materials have long been known to provide adsorptive
properties. The earliest applications may date back centuries with the
discovery that charred materials could be used to remove tastes, colours
and odours from water. Now activated carbons are used widely in
industrial applications which include decolourizing sugar solutions,