Page 20 - Adsorption Technology & Design, Elsevier (1998)
P. 20
Adsorbents 17
because they are closed at both ends. Control of the pore sizes and of their
distribution in the manufacturing process allows a broad range of adsorbents
to be available offering widely differing selectivities. Carbons for gas phase
applications require smaller pores while carbons for liquid phase ap-
plications tend to have larger pore diameters, of the order of 3 nm or larger.
Carbons for liquid phase applications also need to be made with surfaces of
the appropriate wettability.
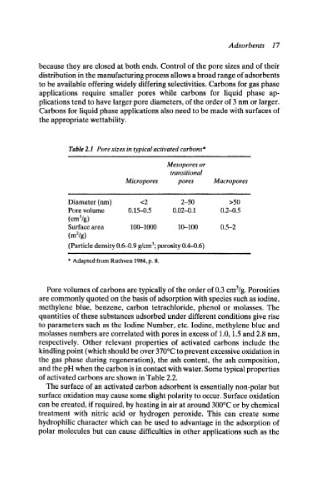
Table 2.1 Pore sizes in typical activated carbons*
iii iii i i iii i ii i
Mesopores or
transitional
Micropores pores Macropores
, , ,, i i ii i,
Diameter (nm) <2 2-50 >50
Pore volume 0.15--0.5 0.02--0.1 0.2--0.5
(cma/g)
Surface area 100--1000 10--100 0.5-2
(m2/g)
(Particle density 0.6--0.9 g/cm3; porosity 0.4--0.6)
iii i i i ii i ii iiii ii i i i i i i
* Adapted from Ruthven 1984, p. 8.
Pore volumes of carbons are typically of the order of 0.3 cm3/g. Porosities
are commonly quoted on the basis of adsorption with species such as iodine,
methylene blue, benzene, carbon tetrachloride, phenol or molasses. The
quantities of these substances adsorbed under different conditions give rise
to parameters such as the Iodine Number, etc. Iodine, methylene blue and
molasses numbers are correlated with pores in excess of 1.0, 1.5 and 2.8 nm,
respectively. Other relevant properties of activated carbons include the
kindling point (which should be over 370~ to prevent excessive oxidation in
the gas phase during regeneration), the ash content, the ash composition,
and the pH when the carbon is in contact with water. Some typical properties
of activated carbons are shown in Table 2.2.
The surface of an activated carbon adsorbent is essentially non-polar but
surface oxidation may cause some slight polarity to occur. Surface oxidation
can be created, if required, by heating in air at around 300~ or by chemical
treatment with nitric acid or hydrogen peroxide. This can create some
hydrophilic character which can be used to advantage in the adsorption of
polar molecules but can cause difficulties in other applications such as the