Page 21 - Adsorption Technology & Design, Elsevier (1998)
P. 21
18 Adsorbents
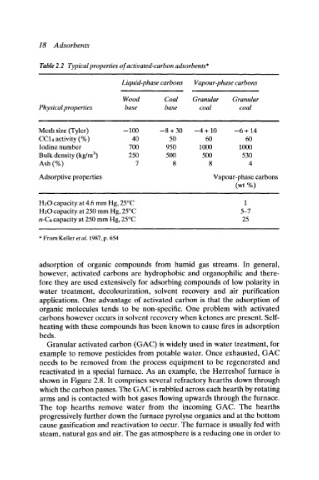
Table 2.2 Typical properties of activated-carbon adsorbents*
Liquid-phase carbons Vapour-phase carbons
Wood Coal Granular Granular
Physical properties base base coal coal
i
Mesh size (Tyler) --100 --8 + 30 -4 + 10 --6+ 14
CCI 4 activity (%) 40 50 60 60
Iodine number 700 950 1000 1000
Bulk density (kg/m 3) 250 500 500 530
Ash (%) 7 8 8 4
Adsorptive properties Vapour-phase carbons
(wt %)
,,
H20 capacity at 4.6 mm Hg, 25~ 1
H20 capacity at 250 mm Hg, 25~ 5-7
n-C4 capacity at 250 mm Hg, 25~ 25
* From Keller et al. 1987, p. 654
adsorption of organic compounds from humid gas streams. In general,
however, activated carbons are hydrophobic and organophilic and there-
fore they are used extensively for adsorbing compounds of low polarity in
water treatment, decolourization, solvent recovery and air purification
applications. One advantage of activated carbon is that the adsorption of
organic molecules tends to be non-specific. One problem with activated
carbons however occurs in solvent recovery when ketones are present. Self-
heating with these compounds has been known to cause fires in adsorption
beds.
Granular activated carbon (GAC) is widely used in water treatment, for
example to remove pesticides from potable water. Once exhausted, GAC
needs to be removed from the process equipment to be regenerated and
reactivated in a special furnace. As an example, the Herreshof furnace is
shown in Figure 2.8. It comprises several refractory hearths down through
which the carbon passes. The GAC is rabbled across each hearth by rotating
arms and is contacted with hot gases flowing upwards through the furnace.
The top hearths remove water from the incoming GAC. The hearths
progressively further down the furnace pyrolyse organics and at the bottom
cause gasification and reactivation to occur. The furnace is usually fed with
steam, natural gas and air. The gas atmosphere is a reducing one in order to