Page 16 - Adsorption Technology & Design, Elsevier (1998)
P. 16
Adsorbents 13
02
q
N2
Amount
adsorbed
per unit
weight of
adsorbent
i .......... I .......... I
p Pressure
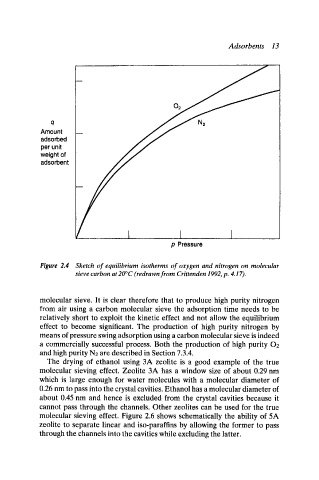
Figure 2.4 Sketch of equilibrium isotherms of oxygen and nitrogen on molecular
sieve carbon at 20~ (redrawn from Crittenden 1992, p. 4.17).
molecular sieve. It is clear therefore that to produce high purity nitrogen
from air using a carbon molecular sieve the adsorption time needs to be
relatively short to exploit the kinetic effect and not allow the equilibrium
effect to become significant. The production of high purity nitrogen by
means of pressure swing adsorption using a carbon molecular sieve is indeed
a commercially successful process. Both the production of high purity 02
and high purity N2 are described in Section 7.3.4.
The drying of ethanol using 3A zeolite is a good example of the true
molecular sieving effect. Zeolite 3A has a window size of about 0.29 nm
which is large enough for water molecules with a molecular diameter of
0.26 nm to pass into the crystal cavities. Ethanol has a molecular diameter of
about 0.45 nm and hence is excluded from the crystal cavities because it
cannot pass through the channels. Other zeolites can be used for the true
molecular sieving effect. Figure 2.6 shows schematically the ability of 5A
zeolite to separate linear and iso-paraffins by allowing the former to pass
through the channels into the cavities while excluding the latter.