Page 15 - Adsorption Technology & Design, Elsevier (1998)
P. 15
12 Adsorbents
More than one mechanism of separation can be exploited in some
applications but in others certain mechanisms can be counterproductive.
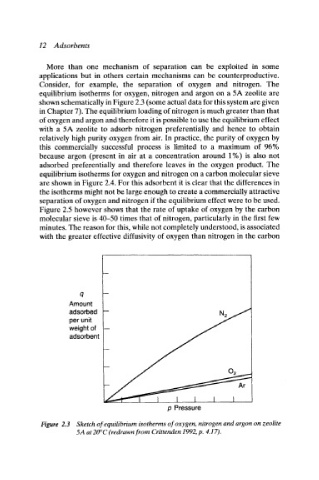
Consider, for example, the separation of oxygen and nitrogen. The
equilibrium isotherms for oxygen, nitrogen and argon on a 5A zeolite are
shown schematically in Figure 2.3 (some actual data for this system are given
in Chapter 7). The equilibrium loading of nitrogen is much greater than that
of oxygen and argon and therefore it is possible to use the equilibrium effect
with a 5A zeolite to adsorb nitrogen preferentially and hence to obtain
relatively high purity oxygen from air. In practice, the purity of oxygen by
this commercially successful process is limited to a maximum of 96%
because argon (present in air at a concentration around 1%) is also not
adsorbed preferentially and therefore leaves in the oxygen product. The
equilibrium isotherms for oxygen and nitrogen on a carbon molecular sieve
are shown in Figure 2.4. For this adsorbent it is clear that the differences in
the isotherms might not be large enough to create a commercially attractive
separation of oxygen and nitrogen if the equilibrium effect were to be used.
Figure 2.5 however shows that the rate of uptake of oxygen by the carbon
molecular sieve is 40-50 times that of nitrogen, particularly in the first few
minutes. The reason for this, while not completely understood, is associated
with the greater effective diffusivity of oxygen than nitrogen in the carbon
q
Amount
adsorbed
N2
per unit
weight of
adsorbent
02
I I I I
p Pressure
Figure 2.3 Sketch of equilibrium isotherms of oxygen, nitrogen and argon on zeolite
5A at 20~ (redrawn from Crittenden 1992, p, 4.17).