Page 153 - Adsorption Technology & Design, Elsevier (1998)
P. 153
Design procedures 141
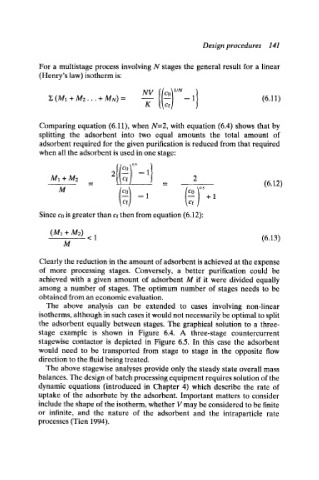
For a multistage process involving N stages the general result for a linear
(Henry's law) isotherm is:
/
]E (M1 + M2... + MN) = --1 (6.11)
K
Comparing equation (6.11), when N=2, with equation (6.4) shows that by
splitting the adsorbent into two equal amounts the total amount of
adsorbent required for the given purification is reduced from that required
when all the adsorbent is used in one stage:
M~ + M2
2 t~ cf ] 2 (6.12)
M
co -- 1 co + 1
Since co is greater than cf then from equation (6.12):
(M1 + ME)
< 1 (6.13)
M
Clearly the reduction in the amount of adsorbent is achieved at the expense
of more processing stages. Conversely, a better purification could be
achieved with a given amount of adsorbent M if it were divided equally
among a number of stages. The optimum number of stages needs to be
obtained from an economic evaluation.
The above analysis can be extended to cases involving non-linear
isotherms, although in such cases it would not necessarily be optimal to split
the adsorbent equally between stages. The graphical solution to a three-
stage example is shown in Figure 6.4. A three-stage countercurrent
stagewise contactor is depicted in Figure 6.5. In this case the adsorbent
would need to be transported from stage to stage in the opposite flow
direction to the fluid being treated.
The above stagewise analyses provide only the steady state overall mass
balances. The design of batch processing equipment requires solution of the
dynamic equations (introduced in Chapter 4) which describe the rate of
uptake of the adsorbate by the adsorbent. Important matters to consider
include the shape of the isotherm, whether V may be considered to be finite
or infinite, and the nature of the adsorbent and the intraparticle rate
processes (Tien 1994).