Page 1023 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1023
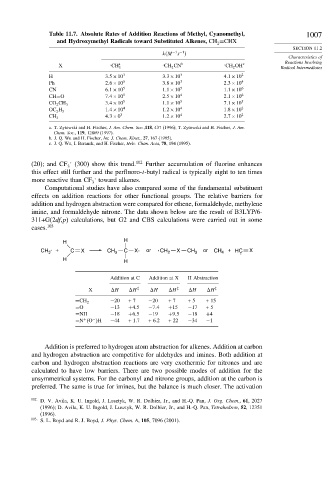
Table 11.7. Absolute Rates of Addition Reactions of Methyl, Cyanomethyl, 1007
and Hydroxymethyl Radicals toward Substituted Alkenes, CH =CHX
2
SECTION 11.2
k M −1 −1
s
Characteristics of
Reactions Involving
X . CH a 3 . CH 2 CN b . CH 2 OH c Radical Intermediates
H 3 5×10 3 3 3×10 3 4 1×10 2
Ph 2 6×10 5 3 8×10 3 2 3×10 4
CN 6 1×10 5 1 1×10 5 1 1×10 6
CH=O 7 4×10 5 2 5×10 4 2 1×10 6
3 4×10 5 1 1×10 5 7 1×10 5
CO 2 CH 3
1 4×10 4 1 2×10 4 1 8×10 2
OC 2 H 5
4 3×0 3 1 2×10 4 2 7×10 2
CH 3
a. T. Zytowski and H. Fischer, J. Am. Chem. Soc.,118, 437 (1996); T. Zytowski and H. Fischer, J. Am.
Chem. Soc., 119, 12869 (1997).
b. J. Q. Wu and H. Fischer, Int. J. Chem. Kinet., 27, 167 (1995).
c. J. Q. Wu, I. Beranek, and H. Fischer, Helv. Chim. Acta, 78, 194 (1995).
. 102
(20); and CF 3 (300) show this trend. Further accumulation of fluorine enhances
this effect still further and the perfluoro-t-butyl radical is typically eight to ten times
.
more reactive than CF 3 toward alkenes.
Computational studies have also compared some of the fundamental substituent
effects on addition reactions for other functional groups. The relative barriers for
addition and hydrogen abstraction were compared for ethene, formaldehyde, methylene
imine, and formaldehyde nitrone. The data shown below are the result of B3LYP/6-
311+G(2df,p) calculations, but G2 and CBS calculations were carried out in some
cases. 103
H H
CH 3 + C X CH 3 C X or CH 2 X CH 3 or CH 4 + HC X
H H
Addition at C Addition at X H Abstraction
X H H ‡ H H ‡ H H ‡
−20 + 7 −20 +7 +5 +15
=CH 2
=O −13 +4 5 −7 4 +15 −17 + 5
=NH −18 +6 5 −19 +9 5 −18 +4
−
+
=N O
H −44 + 1.7 + 6.2 + 22 −34 −1
Addition is preferred to hydrogen atom abstraction for alkenes. Addition at carbon
and hydrogen abstraction are competitive for aldehydes and imines. Both addition at
carbon and hydrogen abstraction reactions are very exothermic for nitrones and are
calculated to have low barriers. There are two possible modes of addition for the
unsymmetrical systems. For the carbonyl and nitrone groups, addition at the carbon is
preferred. The same is true for imines, but the balance is much closer. The activation
102 D. V. Avila, K. U. Ingold, J. Lusztyk, W. R. Dolbier, Jr., and H.-Q. Pan, J. Org. Chem., 61, 2027
(1996); D. Avila, K. U. Ingold, J. Lusztyk, W. R. Dolbier, Jr., and H.-Q. Pan, Tetrahedron, 52, 12351
(1996).
103
S. L. Boyd and R. J. Boyd, J. Phys. Chem. A, 105, 7096 (2001).