Page 1022 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1022
1006 (e.g., OR) retard reaction, whereas EWGs (e.g., CN) accelerate reaction, which is
in agreement with the classification of alkyl radicals as nucleophilic. A relationship
CHAPTER 11
was also found between reactivity and the ground state stabilization of the alkene.
Free Radical Reactions Certain substituent combinations (e.g., CN,CN or Cl,Cl) significantly destabilize the
alkene, and these compounds are highly reactive toward alkyl radicals. On the other
hand, the (OC H CH ) combination stabilizes the alkene and such compounds are
2
3
5
less reactive toward alkyl radicals. These results are consistent with an early TS for
radical addition controlled by SOMO-LUMO interactions. The regiochemistry, which
generally involves addition at the -carbon, also correlates with the coefficient of the
LUMO, as would be expected for an FMO-controlled reaction.
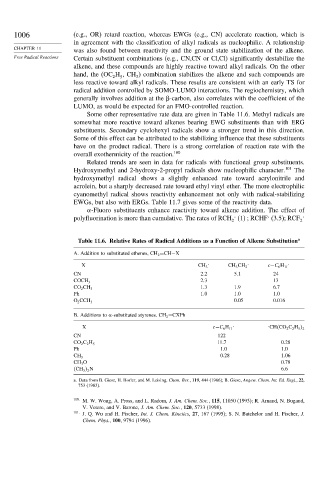
Some other representative rate data are given in Table 11.6. Methyl radicals are
somewhat more reactive toward alkenes bearing EWG substituents than with ERG
substituents. Secondary cyclohexyl radicals show a stronger trend in this direction.
Some of this effect can be attributed to the stabilizing influence that these substituents
have on the product radical. There is a strong correlation of reaction rate with the
overall exothermicity of the reaction. 100
Related trends are seen in data for radicals with functional group substituents.
Hydroxymethyl and 2-hydroxy-2-propyl radicals show nucleophilic character. 101 The
hydroxymethyl radical shows a slightly enhanced rate toward acrylonitrile and
acrolein, but a sharply decreased rate toward ethyl vinyl ether. The more electrophilic
cyanomethyl radical shows reactivity enhancement not only with radical-stabilizing
EWGs, but also with ERGs. Table 11.7 gives some of the reactivity data.
-Fluoro substituents enhance reactivity toward alkene addition. The effect of
. . .
polyfluorination is more than cumulative. The rates of RCH (1) ; RCHF (3.5); RCF
2 2
Table 11.6. Relative Rates of Radical Additions as a Function of Alkene Substitution a
A. Addition to substituted ethenes, CH 2 =CH−X
. . .
X CH 3 CH 3 CH 2 c−C 6 H 11
CN 2.2 5.1 24
2.3 13
COCH 3
1.3 1.9 6.7
CO 2 CH 3
Ph 1.0 1.0 1.0
0.05 0.016
O 2 CCH 3
B. Additions to -substituted styrenes. CH 2 =CXPh
X c−C 6 H 11 . . CH CO 2 C 2 H 5
2
CN 122
11.7 0.28
CO 2 C 2 H 5
Ph 1.0 1.0
0.28 1.06
CH 3
CH 3 O 0.78
CH 3
2 N 6.6
a. Data from B. Giese, H. Horler, and M. Leising, Chem. Ber., 119, 444 (1986); B. Giese, Angew. Chem. Int. Ed. Engl., 22,
753 (1983).
100 M. W. Wong, A. Pross, and L. Radom, J. Am. Chem. Soc., 115, 11050 (1993); R. Arnaud, N. Bugaud,
V. Vetere, and V. Barone, J. Am. Chem. Soc., 120, 5733 (1998).
101
J. Q. Wu and H. Fischer, Int. J. Chem. Kinetics, 27, 167 (1995); S. N. Batchelor and H. Fischer, J.
Chem. Phys., 100, 9794 (1996).