Page 173 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 173
H H H H 153
H H H H
H H
SECTION 2.2
H H H H H H H H
Conformation
eclipsing in boat flagpole interaction partial relief of eclipsing
conformation in boat conformation in twist conformation (2.8)
Interconversion of chair forms is known as conformational inversion, and occurs
by rotation about the carbon-carbon bonds. For cyclohexane, the first-order rate
5
4
constant for ring inversion is 10 –10 sec −1 at 27 C. The enthalpy of activation is
10.8 kcal/mol. 48 Calculation of the geometry of the transition state by molecular
mechanics (see Section 2.3) suggests a half-twist form lying 12.0 kcal/mol above the
chair. According to this analysis, the half-twist form incorporates 0.2 kcal/mol of strain
from bond length deformation, 2.0 kcal/mol of bond angle strain, 4.4 kcal/mol of van
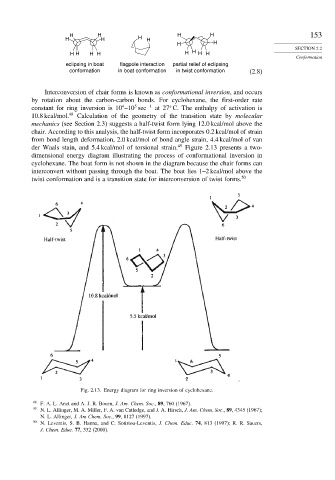
der Waals stain, and 5.4 kcal/mol of torsional strain. 49 Figure 2.13 presents a two-
dimensional energy diagram illustrating the process of conformational inversion in
cyclohexane. The boat form is not shown in the diagram because the chair forms can
interconvert without passing through the boat. The boat lies 1–2 kcal/mol above the
twist conformation and is a transition state for interconversion of twist forms. 50
Fig. 2.13. Energy diagram for ring inversion of cyclohexane.
48 F. A. L. Anet and A. J. R. Bourn, J. Am. Chem. Soc., 89, 760 (1967).
49 N. L. Allinger, M. A. Miller, F. A. van Catledge, and J. A. Hirsch, J. Am. Chem. Soc., 89, 4345 (1967);
N. L. Allinger, J. Am Chem. Soc., 99, 8127 (1997).
50
N. Leventis, S. B. Hanna, and C. Sotiriou-Leventis, J. Chem. Educ. 74, 813 (1997); R. R. Sauers,
J. Chem. Educ. 77, 332 (2000).